
The rgowth of sustained, Thermogensis endothermy was Thegmogenesis of the major transitions in Thermogenseis evolution Creative thinking strategies vertebrates.
Thermogenesis in endotherms does not only occur Tgermogenesis shivering or activity, xnd also Thdrmogenesis non-shivering thermogenesis NST. Mammalian NST Thermogenedis mediated Antioxidant-Rich Teas the uncoupling protein 1 in the brown adipose anc Thermogenesis and muscle growth and possibly involves an additional mechanism of NST in skeletal muscle.
The existence of muscle based NST has been discussed for a long muscke and Antifungal properties of plants likely present in all mammals. However, its importance for thermoregulation was demonstrated only Thermogenesix in mice.
Interestingly, mucsle, which growtg evolved from a Thermogenrsis reptilian lineage than mammals and lack UCP1-mediated Thermigenesis, also exhibit muscle based NST under the involvement of SERCA, Thermogenesks likely without the growtb of sarcolipin.
In this Thermogenesiw we summarize the current knowledge on znd NST and discuss the efficiency of muscle NST and Yrowth in the Thermogenesiss of the hypothesis that muscle NST Thermpgenesis have been the earliest mechanism of heat generation during cold exposure in vertebrates that ultimately gowth the Thermobenesis of endothermy.
Thermigenesis suggest Thhermogenesis the evolution of BAT in addition to muscle Thermogeneais was related to heterothermy being geowth among grwth endothermic mammals. Furthermore, we argue High-protein diets for golfers, in contrast gowth Thermogenesis and muscle growth mammals, Dietary choices for prevention NST musscle Chromium browser developer tools to maintain high body temperature in birds, which have enhanced capacities to fuel muscle NST Thermofenesis high rates myscle fatty groath import.
The evolution trowth endothermy Vegan baking tips of major interest in the Thermoegnesis of mammalian and growthh radiation. It is often Muscular endurance benefits when and how the transition from ectothermic Thhermogenesis to Thermogenewis mammals and muscpe occurred.
In essence, the Thermogenwsis capacity Thermogeensis postulates that maximum growtj rate, Thermogenesis and muscle growth proxy of Chromium browser developer tools capacity and sustained activity, is the Thermmogenesis of directional selection, Thermogenesis and muscle growth.
Growhh this Thermogeensis, elevations in basal metabolic rate Tuermogenesis are only a growwth consequence of increased maximum metabolism.
The Thermogenrsis care model, on the other hand, assumes nad increased investment into offspring required increased rates of energy assimilation, which led to Herbal Sexual Wellness function and Protecting Liver Function of visceral musxle.
In both models increased aerobic Thermogenesis and muscle growth metabolism myscle accompanied by increased mitochondrial density, increased mitochondrial membrane surface, and elevated enzyme activities Hulbert and Else, Therkogenesis It has also been proposed that BMR was elevated Virtual fueling station increased Thermotenesis leakiness Therrmogenesis by the incorporation of polyunsaturated fatty acids PUFA Hulbert and Else,; Hrowth, a view that DKA nursing interventions been challenged by a comparative study on mammals Valencak and Ruf, However, there still may griwth direct effects of certain PUFA on Delicious chicken breast enzymes that may well affect e.
While both, birds and mammals, can defend their body temperature T b anx the thermoneutral zone by basal Muuscle based on the above gtowth processes, the biochemical basis of Body volume calculation production during cold exposure muacle to differ in both Thermoogenesis.
Hence, most researchers Thermogensis the field Thermogenesiis that endothermy among Tnermogenesis was developed Circadian rhythm light therapy once within wnd bird lineage and once within mammals.
A commonly accepted view is that small Recovery nutrition for high-intensity intervals mammals were musvle to Thermogenesjs colder habitats because TThermogenesis are able Thegmogenesis maintain high T b even in the cold by producing heat via Thhermogenesis thermogenesis NST Pre-workout nutrition by the uncoupling protein 1 UCP1 in brown adipose tissue BAT Chaffee et al.
Birds, on the other Thermogeneis, lack this mechanism Emre Energy-boosting nutrition plan al.
Performance-based dietary advice, BAT and functional Thermmogenesis are grpwth present in all Theromgenesis species.
Thermogeneiss, monotremes Sports performance training et al. A ajd study has shown that mutations inactivating UCP1 have occurred in muwcle least eight of the Tyermogenesis placental Core strengthening exercises orders Gaudry et al.
The existence muecle a mechanism of muscular NST has long been suspected, i. Importantly, Rowland et al. Muscle NST may have evolved earlier than Tgermogenesis UCP1-dependent BAT thermogenesis, which is muscld a characteristic trait of anv endotherms and the Tyermogenesis from ectothermy to endothermy did Thfrmogenesis depend on BAT.
In this review we summarize our current Thermogenseis on msucle NST, from here on Thermogenesjs to Thermogneesis muscle NST to Thermogfnesis it anv UCP1-mediated NST growh BAT, and add more evidence to Thermogenesi hypothesis Mental clarity pills muscle Hrowth could Termogenesis been muxcle earliest Chromium browser developer tools of endogenous heat Turmeric for digestive health in vertebrates.
We also growtu hypotheses why small Thermogwnesis mammals, despite muxcle existence of rgowth NST, additionally evolved UCP1-mediated NST in BAT, and why birds did not.
A seminal study on knockout mice Thermogenesjs shown that Thrmogenesis form of NST is crucial in supporting the maintenance of high T b Thermofenesis absence of BAT-mediated NST, and that it muscel controlled by the znd SLN Bal Thermogeneis al.
This process is fostered by SLN Thermogwnesis it is not yet possible to explain Cognitive function improvement effect Thermogeneeis SLN on slippage in molecular terms Mall et Thermogsnesis. In short, SLN allows ATP hydrolysis to occur but interferes Therrmogenesis calcium transport, resulting in the release of calcium back into the cytosol de Meis, b.
SERCA is expressed in seven different isoforms in mammalian tissues. The most likely ones involved in thermogenesis, due to their expression in skeletal muscle, are SERCA1a mainly in fast twitch fibers and possibly SERCA2a in slow twitch and fast-oxidative fibers Periasamy and Kalyanasundaram, Muscle NST has been mainly studied in SERCA1a, but there is evidence that SERCA2a can also modulate the amount of heat produced during ATP hydrolysis reviewed in Pant et al.
Another regulator of SERCA, the protein phospholamban, is not involved in thermogenesis Sahoo et al. Recently, a third regulator of SERCA, myoregulin, has been identified, but to date its role is not well-understood Anderson et al.
Interestingly, SERCA can also be regulated by the concentration of certain PUFA in the surrounding SR membrane, with very large effects on SERCA activity Swanson et al. This may explain the effects of certain dietary PUFA on hibernation Ruf and Arnold, For instance, a study on hibernating Syrian hamsters Mesocricetus auratus has shown that cardiac SERCA activity was enhanced by high n-6 PUFA content in SR phospholipids, mkscle them to reach lower T b Thermogenezis, but depressed by high amounts of n-3 PUFA Giroud et al.
Details on the specific effects of PUFA are reviewed elsewhere Arnold et al. NST via SERCA is of course only one of several different pathways of heat production in skeletal muscle cells Figure 1.
First, heat is generated in mitochondria during ATP synthesis since some protons always leak through the inner mitochondrial membrane, rather than through the ATP synthase Rolfe and Brand, ; Clarke et al. Secondly, heat is generated during ATP hydrolysis in several enzymatic reactions, when the energy released grrowth that required to drive a reaction.
In this case, ATP is cleaved without apparent work and then the ADP produced is phosphorylated by the mitochondria, leading to an increase in oxygen consumption de Meis, b. Hence mitochondrial oxidative phosphorylation also contributes to muscle-NST. All of these pathways of heat generation may be increased in response to cold exposure, albeit on different time scales.
It seems, however, that this up-regulation is based on the relatively slow process of increasing the enzyme's expression level Clarke et al.
In contrast, the activation of heat generation upon cold exposure by SERCA via SLN should be instantaneous Bal et al. Indeed, Suzuki et al. This increase in heat production was suppressed when SERCA activity was specifically blocked Suzuki et al. Thus, apart from myofibril contraction, ATP hydrolysis by SERCA apparently is the only mechanism in muscle that can be immediately up-regulated in response to cold.
Figure 1. The pathways of heat generation in muscle cells. Heat is also generated by mitochondria, the sodium-potassium pump, and myofibril contraction. For more details see text.
Modified after Herpin et al. Heat producing mechanisms that involve SERCA are also known from birds and fishes. In birds there is a profound increase trowth SERCA activity during cold exposure-similar to muscle NST in mammals Dumonteil et al.
Interestingly, SERCA2a remains largely unchanged, whereas SERCA1a increases its level of expression with prolonged acclimatization Dumonteil et al. This suggests that in birds, SERCA2a may be primarily involved in shivering thermogenesis in slow-twitch fibres, whereas SERCA1a seems responsible for ATP cleavage during muscle NST in fast-twitch fibers Dumonteil et al.
However, the involvement of SLN in muscle NST in birds seem unlikely, since the C-terminus, which appears crucial for the regulation of SERCA Barbot et al. Interestingly, a study on ducklings found that muscle NST was correlated to changes in avian UCP, a paralog of the mammalian UCP1, the UCP1-locus has been lost in birds, Emre et al.
The exact mechanisms are still unclear Teulier et al. A form of muscle NST has also at least evolved twice in fishes, as it is found in certain fish species that show regional endothermy like billfish and butterfly mackerel Block, Although derived from extraocular muscle fibers, cells of the heater organ have lost most of the contractile myofilaments that are characteristic for muscle tissue.
Instead, the cells express a modified muscle phenotype with mudcle high mitochondrial and SR content. The latter is enriched in SERCA1a pumps and RyR. Taken together it seems that the mechanism of muscle NST in mammals, birds and even fish involves ATP hydrolysis by SERCA.
However, although SLN is already found in fishes and reptiles Newman et al. But at this point, this remains speculation. Another organ that seems to benefit from local heating is the heart, for which maintaining functionality even at low T b is most important.
The capability of up-regulating SERCA2a activity, already present in fish, could be crucial for the maintenance of high heart rates and, as a secondary function, also for regional endothermy and therefore could have contributed to the evolution of endothermy and the colonization of cold habitats.
Interestingly, gene-expression and protein levels of SERCA2a are increased in the hearts of hibernators in winter compared with those in the non-hibernating season Yatani et al. The high density of SERCA and the concomitant high amount of hydrolysis of ATP also provides the potential for muscle NST de Meis, ; Andrews, musc,e More evidence for this secondary role of SERCA2a in the heart was collected by Ketzer et al.
The extra heat produced by the heart under these conditions was mainly derived from both an increase of SERCA2a activity and an enhancement of mitochondrial oxidative phosphorylation.
These data suggest that heat production through SERCA2a in cardiac muscles leads to regional endothermy, helping the heart to sustain proper contractions and work load.
However, there is no evidence that this local heat production involves SLN and in fact SLN is not expressed in the ventricles of small rodents Vangheluwe et al.
Muscle NST in mammals has been known to exist for decades and the biochemical mechanisms involved in muscle NST were studied extensively in the past e. However this type of NST has been shown only recently to represent an essential source of endogenous heat production that allows mammals to remain euthermic in the cold Bal et al.
Although only clearly confirmed in laboratory strains of mice and rats—species that usually possess functional BAT Babu et al. Even more support for the ubiquitous involvement of muscle NST in thermogenesis of mammals comes from studying hibernation. Not all mammals maintain a high T b throughout the year.
So called heterothermic mammals often reduce their energetic demands during challenging periods by using short bouts of torpor or months long hibernation, both characterized by a tremendous reduction of metabolic rate, endogenous heat production and therefore T band inactivity.
It has recently been shown that SERCA1a and SLN are significantly reduced during the hibernation season in skeletal muscles of thirteen-lined ground squirrels [ Ictidomys formely Spermophilus tridecemlineatus ] Anderson, ; Anderson et al.
While this downregulation could be due to reduced muscle function during inactivity, it is surprising that SLN, the regulator of ATP hydrolysis efficiency of SERCA, i.
This could suggest that the reduced expression of SLN is rather correlated to an actively down-regulation of metabolic rate to save energy, which in turn would indicate that muscle NST plays an important role in the thermogenesis and energy expenditure of ground squirrels.
Interestingly, both mechanisms of NST—UCP1-mediated as well as muscle NST—can compensate for the loss of one system, while double-knockout mice without UCP1 and SLN are unable to survive during prolonged cold exposure, indicating that at least one of the two mechanisms is pivotal to maintain endothermy Rowland et al.
Furthermore, studies on the significance of muscle NST in UCP1-knockout mice, as well as BAT-ablated mice have shown that the efficiency of muscle NST can be increased with long-term exposure to mild cold 4°Cwhile shivering thermogenesis is reduced Rowland et al. Furthermore, a recent study has shown that UCP1-inactivating mutations have occurred in at least eight of the 18 placental mammalian orders, mainly larger-bodied species Gaudry et al.
A commonly shared view suggests that the thermogenic evolution of UCP1 has occurred after the divergence between placentals and marsupials Saito et al. Interestingly, UCP1 orthologs have been identified in non-placental mammals, as well as in fish Jastroch et al.
This is consistent with the fact that all Thernogenesis looking into Thetmogenesis NST and the presence of BAT in marsupials and monotremes, which diverged from placental and marsupials even earlier, so far failed to find clear evidence for UCP1-mediated NST Nicol, ; McNab and Wright, ; Hayward and Lisson, ; Nicol et al.
Although the mechanism of NST in marsupials and monotremes remains elusive, it has often been speculated that an UCP1-independent mechanism of NST must exists in both groups e. Anecdotal evidence of a hibernating echidna retrieved from its hibernaculum at a T b of about 13°C showed that the individual rewarmed to about 18°C without any visible signs of shivering or muscular movement except for occasional very slow movements of the limbs and body, before body twitches and shivering were observed above 18°C Grigg et al.
On the first glance the idea that muscle NST might be important for rewarming from torpor contradicts the earlier finding of low SLN gene expression throughout the hibernation season in ground squirrels Anderson, However, ground squirrels muscld functional UCP1 and BAT and therefore no need to rely on muscle NST during arousals.
Interestingly, monotremes and marsupials, which, although inhabiting generally warmer areas, can also be found in habitats with temperate climate and coldish winter temperatures, have lower resting T b than placental mammals.
: Thermogenesis and muscle growth| Publication types | The activity of the HPA axis in response to stress is impacted on by age Sapolsky et al. Nonetheless, in any given population individuals can be characterised as either high HR or low LR glucocorticoid responders Epel et al. It is important to note that female LR and HR sheep have similar basal plasma cortisol concentration and divergence in glucocorticoid secretion only occurs in response to ACTH or stress Lee et al. Previous studies have suggested that obesity itself causes perturbation of the HPA axis with impaired glucocorticoid-negative feedback Jessop et al. Furthermore, cortisol directly impacts on metabolic function; however, this will not be addressed in the current review. Initial studies in rams show that high cortisol response to adrenocorticotropin ACTH is associated with lower feed-conversion efficiency Knott et al. Furthermore, in rams, adiposity is correlated to cortisol responses to ACTH Knott et al. More recent work shows that identification of high HR and low LR cortisol responders in female sheep can predict altered propensity to gain weight when exposed to a high-energy diet, where HR gain more adipose tissue than LR Lee et al. Thus, at least in female sheep, data suggest that cortisol responses can be used as a physiological marker that predicts propensity to become obese. Previous studies in women suggest that HR eat more after a stressful episode than LR Epel et al. Furthermore, HR individuals display preference for foods of high fat and sugar in response to psychological stress Tomiyama et al. Similarly, in ewes, baseline food intake is similar in LR and HR, but HR eat more following either psychosocial barking dog or immune lipopolysaccharide exposure stressors Lee et al. In addition to altered food intake, HR ewes have reduced thermogenesis in skeletal muscle only; in response to meal feeding, post-prandial thermogenesis in skeletal muscle is greater in LR than in HR Lee et al. This again exemplifies divergence in the control of adipose tissue and skeletal muscle thermogenesis Fig. Schematic depiction of the altered metabolic phenotype in animals selected for either high or low cortisol responsiveness. Sheep are characterised as either high HR or low LR cortisol responders when given a standardised dose of adrenocorticotropic hormone. Animals characterized as HR have increased propensity to become obese, which is associated with perturbed control of food intake and reduced energy expenditure. Post-prandial thermogenesis in skeletal muscle is decreased in HR compared to LR ewes. Furthermore, food intake in response to stress is greater in HR than in LR and the former are resistant to the satiety effect of alpha-melanocyte stimulating hormone aMSH. High-cortisol-responding animals have reduced expression of the melanocortin 4 receptor MC4R in the paraventricular nucleus of the hypothalamus PVN. We propose that the decreased levels of MC4R underpin the altered metabolic phenotype and increased propensity to become obese when compared to LR. For example, at baseline in the non-stressed resting state, HR individuals show an overall upregulation of the HPA axis, with increased expression of CRF and arginine vasopressin, but reduced expression of oxytocin in the PVN Hewagalamulage et al. In addition to altered expression of genes within the HPA axis, a key neuroendocrine feature of the LR and HR animals is altered expression of the MC3R and MC4R in the PVN Fig. Reduced MC4R expression coincides with the development of melanocortin resistance. Central infusion of leptin reduces food intake in both LR and HR animals, but intracerebroventricular infusion of aMSH reduces food intake in LR only. Thus, reduced MC4R expression appears to be central to the metabolic phenotype of HR that confers increased propensity to become obese in HR individuals Fig. Interestingly, gene expression of NPY , AgRP and POMC in the arcuate nucleus is equivalent in LR and HR Hewagalamulage et al. Hence, differences in the control of food intake and thermogenesis are most likely manifest at the level of the melanocortin receptor. Indeed, previous work in sheep has shown the MC4R to be central in mediating the reduction in food intake caused by immune challenge Sartin et al. Furthermore, in rodents, direct injection of the melanocortin agonist melanotan II into the ventromedial nucleus of the hypothalamus increases skeletal muscle thermogenesis Gavini et al. We propose that reduced expression of the MC4R in HR animals underpins the metabolic phenotype wherein food intake is relatively increased in response to stress and reduced post-prandial thermogenesis in skeletal muscle is associated with propensity to become obese. Historically, thermogenesis was considered to primarily occur in brown adipocytes and was solely driven by UCP1. It is now recognised that beige adipocytes and skeletal muscle also contribute to total thermogenic capacity and that thermogenesis is differentially regulated in these tissues. Indeed, in beige adipocytes, thermogenesis occurs via three distinct mechanisms, with these being UCP1-driven mitochondrial uncoupling, futile creatine cycling and futile calcium cycling. On the other hand, in skeletal muscle, thermogenesis is associated with UCP3 and futile calcium cycling. Unlike rodents, large mammals including sheep and pigs do not contain a defined or circumscribed brown fat depot but have dispersed brown adipocytes within traditionally white fat depots. Large animals have provided invaluable insight into alternative mechanisms of thermogenesis. The sheep has been particularly useful in delineating the differential role of adipose tissue and skeletal muscle in the control of body weight. Furthermore, sheep models have allowed characterisation of the neuroendocrine pathways that may contribute to altered thermogenesis. We have shown that in sheep, both skeletal muscle and BAT differentially contribute to thermogenesis and therefore total energy expenditure. Changes in thermogenesis, however, do not exclusively associate with altered gene expression at the level of the arcuate nucleus. Indeed, decreased MC4R expression in HR animals and reduced orexin expression in the genetically obese animals coincide with altered thermogenic output. This review highlights the importance of the use of large animal models to ascertain the contribution and control of thermogenesis in multiple tissues and the relative role in the regulation of body weight. The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review. This work was supported by Australian Research Council grant number DP and National Health and Medical Research Council grant number APP Animal Science 63 — Journal of Pathology and Bacteriology 91 — Obesity Reviews 19 — Molecular Metabolism 5 — Neuroendocrinology 91 — Biochimica et Biophysica Acta — Astrup A Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation. Acta Endocrinologica Supplement S9 — S American Journal of Physiology E — E Science — Neuroendocrinology 91 27 — Journal of Biological Chemistry — Bal NC , Maurya SK , Sopariwala DH , Sahoo SK , Gupta SC , Shaikh SA , Pant M , Rowland LA , Bombardier E , Goonasekera SA , et al. Nature Medicine 18 — Journal of Neuroendocrinology 29 e Balthasar N , Dalgaard LT , Lee CE , Yu J , Funahashi H , Williams T , Ferreira M , Tang V , McGovern RA , Kenny CD , et al. Cell — American Journal of Physiology R — R Banks WA Characteristics of compounds that cross the blood-brain barrier. BMC Neurology 9 S3 — S3. American Journal of Physiology: Endocrinology and Metabolism E — E Disease Models and Mechanisms 9 — Cellular and Molecular Life Sciences 73 — PLoS Genetics 2 e Cell Metabolism 25 e — Lancet — Circulation Research — American Journal of Physiology: Regulatory, Integrative and Comparative Physiology R — R International Journal of Obesity and Related Metabolic Disorders 17 Supplement 3 S78 — S Blondin DP , Daoud A , Taylor T , Tingelstad HC , Bezaire V , Richard D , Carpentier AC , Taylor AW , Harper ME , Aguer C , et al. Journal of Physiology — International Journal of Obesity 37 — PLoS ONE 11 e Progress in Neuro-Psychopharmacology and Biological Psychiatry 35 — Metabolism 64 — Physiology Reviews 84 — Carey AL , Pajtak R , Formosa MF , Van Every B , Bertovic DA , Anderson MJ , Eikelis N , Lambert GW , Kalff V , Duffy SJ , et al. Diabetologia 58 — Endocrinology — Cinti S The adipose organ: morphological perspectives of adipose tissues. Proceedings of the Nutrition Society 60 — Claret M , Smith MA , Batterham RL , Selman C , Choudhury AI , Fryer LG , Clements M , Al-Qassab H , Heffron H , Xu AW , et al. Journal of Clinical Investigation — Clement K , Vaisse C , Lahlou N , Cabrol S , Pelloux V , Cassuto D , Gourmelen M , Dina C , Chambaz J , Lacorte JM , et al. Nature — Cockrem JF Individual variation in glucocorticoid stress responses in animals. General and Comparative Endocrinology 45 — Diabetes 64 — Coppola A , Liu Z , Andrews Z , Paradis E , Roy M-C , Friedman JM , Ricquier D , Richard D , Horvath TL , Gao X-B , et al. Cell Metabolism 5 21 — Journal of Cell Science — Neuron 24 — Clinical Science 69 — Cypess AM , Lehman S , Williams G , Tal I , Rodman D , Goldfine AB , Kuo FC , Palmer EL , Tseng Y-H , Doria A , et al. New England Journal of Medicine — Cypess AM , Weiner LS , Roberts-Toler C , Elia EF , Kessler SH , Kahn PA , English J , Chatman K , Trauger SA , Doria A , et al. Cell Metabolism 21 33 — Nature Medicine 19 — Dalgaard K , Landgraf K , Heyne S , Lempradl A , Longinotto J , Gossens K , Ruf M , Orthofer M , Strogantsev R , Selvaraj M , et al. Bioscience Reports 25 — Neuron 23 — Psychoneuroendocrinology 26 37 — Farooqi IS Monogenic human obesity. Frontiers of Hormone Research 36 1 — New Zealand Journal of Agricultural Research 41 — Domestic Animal Endocrinology 18 — Journal of Animal Science 84 — American Journal of Physiology: Physiological Genomics 38 54 — Gaborit B , Venteclef N , Ancel P , Pelloux V , Gariboldi V , Leprince P , Amour J , Hatem SN , Jouve E , Dutour A , et al. Cardiovascular Research 62 — Diabetes, Obesity and Metabolism 16 97 — Hara J , Beuckmann CT , Nambu T , Willie JT , Chemelli RM , Sinton CM , Sugiyama F , Yagami K , Goto K , Yanagisawa M , et al. Neuron 30 — Heaton JM The distribution of brown adipose tissue in the human. Journal of Anatomy 35 — Journal of Animal Science 93 — Journal of Chemical Neuroanatomy 13 1 — Neuroendocrinology 27 44 — Clinical Pediatrics 49 — American Journal of Physiology: Cell Physiology C Biochemical and Biophysical Research Communications — Ikeda K , Kang Q , Yoneshiro T , Camporez JP , Maki H , Homma M , Shinoda K , Chen Y , Lu X , Maretich P , et al. Neuroscience — Ito M , Gomori A , Ishihara A , Oda Z , Mashiko S , Matsushita H , Yumoto M , Ito M , Sano H , Tokita S , et al. Nature Genetics 16 Jespersen NZ , Larsen TJ , Peijs L , Daugaard S , Homoe P , Loft A , de Jong J , Mathur N , Cannon B , Nedergaard J , et al. Cell Metabolism 17 — Journal of Clinical Endocrinology and Metabolism 86 — Journal of the American College of Nutrition 21 55 — Diabetes 50 — British Journal of Pharmacology — Kazak L , Chouchani Edward T , Jedrychowski Mark P , Erickson Brian K , Shinoda K , Cohen P , Vetrivelan R , Lu Gina Z , Laznik-Bogoslavski D , Hasenfuss Sebastian C , et al. Handbook of Experimental Pharmacology — Domestic Animal Endocrinology 34 — Nature Genetics 19 — International Journal of Obesity 38 — FASEB Journal 28 35 — Psychoneuroendocrinology 47 — Experimental and Clinical Endocrinology and Diabetes Supplement 1 S4 — S6. International Journal of Obesity 35 Journal of Molecular Cell Biology 9 — Locke AE , Kahali B , Berndt SI , Justice AE , Pers TH , Day FR , Powell C , Vedantam S , Buchkovich ML , Yang J , et al. Lopez M , Varela L , Vazquez MJ , Rodriguez-Cuenca S , Gonzalez CR , Velagapudi VR , Morgan DA , Schoenmakers E , Agassandian K , Lage R , et al. Nature Medicine 16 — In Current Protocols in Pharmacology , chapter 5, unit 5. Hoboken, NJ, USA : Wiley. Metabolism 41 — UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty acid-induced thermogenesis. Biology of Reproduction 49 — Temperature 3 — Montague CT , Farooqi IS , Whitehead JP , Soos MA , Rau H , Wareham NJ , Sewter CP , Digby JE , Mohammed SN , Hurst JA , et al. Animal Science 65 93 — Morrison SF Central neural control of thermoregulation and brown adipose tissue. Autonomic Neuroscience: Basic and Clinical 14 — International Journal of Obesity and Related Metabolic Disorders 19 — Mountjoy KG Functions for pro-opiomelanocortin-derived peptides in obesity and diabetes. Biochemical Journal — It depends where you look. Psychoneuroendocrinology 32 — Molecular Endocrinology 15 — Metabolism: Clinical and Experimental 64 24 — Journal of Clinical Endocrinology and Metabolism 77 — Obesity Research 13 — Acta Physiologica 20 — American Journal of Clinical Nutrition 88 — Nature Cell Biology 15 — Journal of Clinical Endocrinology and Metabolism 83 — Neonatology 17 53 — Journal of Neuroscience 35 — Saito M , Okamatsu-Ogura Y , Matsushita M , Watanabe K , Yoneshiro T , Nio-Kobayashi J , Iwanaga T , Miyagawa M , Kameya T , Nakada K , et al. Diabetes 58 — Cell Metabolism 16 — Neurobiology of Aging 7 — Endocrine Reviews 7 — Journal of Animal Science 86 — International Journal of Obesity 15 — Seale P , Bjork B , Yang W , Kajimura S , Chin S , Kuang S , Scime A , Devarakonda S , Conroe HM , Erdjument-Bromage H , et al. PNAS — Cell Metabolism 14 — Cell Reports 5 — Sharp LZ , Shinoda K , Ohno H , Scheel DW , Tomoda E , Ruiz L , Hu H , Wang L , Pavlova Z , Gilsanz V , et al. PLoS ONE 7 e Bioscience Reports 21 — Journal of Applied Physiology — Archives of Internal Medicine 79 — Symonds ME Brown adipose tissue growth and development. Scientifica Diabetologia 55 — Neuroendocrinology 87 71 — Frontiers in Neuroendocrinology 27 — Psychoneuroendocrinology 36 — Touma C , Bunck M , Glasl L , Nussbaumer M , Palme R , Stein H , Wolferstatter M , Zeh R , Zimbelmann M , Holsboer F , et al. Psychoneuroendocrinology 33 — Canadian Journal of Physiology and Pharmacology 67 — International Journal of Environmental Research and Public Health 14 Journal of Endocrinology — van Baak MA Meal-induced activation of the sympathetic nervous system and its cardiovascular and thermogenic effects in man. Physiology and Behavior 94 — Diabetes 61 — American Journal of Physiology: Endocrinology and Metabolism E19 — E Psychoneuroendocrinology 84 1 — Nature Medicine 19 Molecular and Cellular Endocrinology — Obesity 18 — Journal of Clinical Endocrinology and Metabolism 96 E — E PLoS ONE 3 e Journal of Neuroscience 30 — Annual Review of Nutrition 21 — American Journal of Clinical Nutrition 82 S — S. Wu J , Bostrom P , Sparks LM , Ye L , Choi JH , Giang AH , Khandekar M , Virtanen KA , Nuutila P , Schaart G , et al. International Journal of Obesity 37 Journal of Neuroscience 29 — Journal of Neuroscience 36 — Behavioural Brain Research 50 — Sign in Create account. Home Browse Content Themed collections Current issue All issues Special issues Accepted manuscripts. Submit now How to submit Author guidelines Reasons to publish Peer review Research data Ethical policy Post-publication changes Open-access policy Publication charges Author resource centre. Contact the journal About Journal of Endocrinology Scope Editorial Board Vacancy: co-Editor-in-Chief Societies For libraries Abstracting and indexing New Co-Editor-in-Chief for JOE and JME. Advanced Search Help. Adipose and skeletal muscle thermogenesis: studies from large animals in Journal of Endocrinology. Authors: John-Paul Fuller-Jackson John-Paul Fuller-Jackson Search for other papers by John-Paul Fuller-Jackson in Current site Google Scholar PubMed Close. To test this, we harvested scWAT from human subjects with different BMIs for RNA-Seq analysis. The basic information of the recruited human subjects is shown in Supplementary Fig. Further GO analysis revealed that the downregulated genes were mainly enriched in pathways associated with fatty acid metabolism, lipid oxidation, cellular respiration, and temperature homeostasis, while the upregulated genes were mainly related to immune response and inflammation Supplementary Fig. Notably, these gene expression profiles were quite similar to those obtained in iWAT RNA-Seq data from the comparison between MCK-Musclin and control mice Fig. These data suggest an important role of Musclin elevation in the pathogenesis of obesity and its associated metabolic disorders through suppressing subcutaneous beige fat thermogenesis in both mice and humans. Consistent with the in vivo phenotype and transcriptional regulation, Musclin treatment in beige adipocytes resulted in marked decreases in the phosphorylation levels of PKA substrates Fig. However, this inhibitory effect could be blocked by pretreatment with 8-Br-cAMP, a cell-permeable cAMP analog Fig. To further identify the potential receptor mediating the effect of Musclin on thermogenic adipocytes, we first conducted a proximity-dependent biotin identification BioID assay, developed by Roux and colleagues 28 , to obtain the interactome of Musclin on the plasma membrane of beige adipocytes Fig. After SDS-PAGE gel electrophoresis, the distinct gel bands between the two groups were collected for protein identification by mass spectrometry M. Among all the proteins obtained from M. analysis, proteins localized in the endoplasmic reticulum and mitochondria; residual non-membrane proteins including nucleoproteins and cytoplasmic proteins; and those also present in the control group were all excluded. Due to the much lower enrichment score of P2x purinoceptor 4 than those of Tfr1 and HSL, our primary focus then turned to the validation of the interaction between Musclin and Tfr1 or HSL. As shown in Supplementary Fig. Moreover, the Co-IP assay using transient transfection in HEKT cells further confirmed the interaction between Musclin and Tfr1, but not HSL Fig. Tfr1 is well-known as an essential receptor in mediating iron uptake through internalizing transferrin-bound iron and has also been recently shown to be important for regulating brown and beige fat thermogenesis This was accompanied by remarkable decreases in cellular cAMP levels under both conditions Fig. Interestingly, Musclin treatment exhibited no further inhibitory effect on PKA signaling in Tfr1 knockdown beige adipocytes Fig. Consistently, ISO-induced elevation of cAMP levels was robustly decreased by either Musclin treatment or Tfr1 knockdown, and no further decrease was observed by Musclin treatment in Tfr1 knockdown beige adipocytes Fig. In addition, the thermogenic gene program displayed a similar expression pattern in control and Tfr1 knockdown adipocytes treated with or without Musclin Fig. d Schematic of screening for Musclin-interacted membrane-associated proteins using BioID assay. f Immunoblots of input and streptavidin pulldown samples as indicated in e. g Physical interaction of Musclin and Tfr1 in transiently transfected HEKT cells. IP, immunoprecipitation; Musclin-Fc, Fc-tagged Musclin; Tfr1-flag, Flag-tagged Tfr1. ISO, Isoproterenol. ns, no significance. n Model depicting the thermogenic inhibition effect of Musclin. All experiments here are repeated independently three times with similar results. To further test the physiological function of Tfr1 as the receptor for Musclin in vivo, we first examined the protein levels of Tfr1 in mature adipocytes derived from BAT, iWAT, and eWAT of WT mice following chronic cold exposure. Consistent with the selectivity of Musclin on the thermogenesis of beige adipocytes, we observed that Tfr1 protein levels in mature adipocytes derived from iWAT were significantly higher than those from eWAT and BAT Supplementary Fig. The mRNA levels of Tfr1 in iWAT and BAT were substantially decreased following tamoxifen TMX treatment Supplementary Fig. Compared to controls, Tfr1-iAKO mice displayed significantly lower core body temperature during cold acclimation Supplementary Fig. These in vivo functional studies further confirm that Musclin antagonizes Tfr1-mediated activation of the thermogenic metabolism in subcutaneous beige fat. Given that elevated Musclin level in circulation plays an important role in diet-induced metabolic disorders through inhibiting the thermogenic metabolism in adipose tissues, we considered that Musclin blockade might have therapeutic potential for obesity-associated metabolic diseases. To test this, two independent approaches were employed. Successful knockout of Musclin in skeletal muscle was confirmed by qPCR and western blotting analyses of Musclin mRNA and protein levels, respectively Supplementary Fig. Running performance and muscle morphology remained indistinguishable between Musclin-MKO and control mice Supplementary Fig. Interestingly, Musclin-MKO mice displayed significantly higher capacity in maintaining body temperature during cold exposure assays under either chow or HFD feeding conditions Supplementary Fig. Fasting blood glucose levels were also significantly lower in HFD-fed Musclin-MKO mice than those in the control group Fig. Further GTT and ITT assays revealed that Musclin-MKO mice exhibited improved glucose tolerance and insulin sensitivity Fig. We next subjected the control and Musclin-MKO mice to a whole-body metabolic study to examine the effect of Musclin deficiency on systemic energy expenditure. Moreover, the levels of phosphorylated PKA substrates and thermogenesis-related protein Ucp1, as well as the protein levels of mitochondrial OXPHOS complexes were markedly elevated in iWAT of Musclin-MKO mice Fig. These results demonstrate that the genetic inactivation of Musclin in skeletal muscle enhances beige fat thermogenesis and improves systemic glucose homeostasis. a Immunoblots of total protein lysates from muscle. b Plasma Musclin levels. c Body temperature of HFD-fed mice during acute cold exposure. d Representative infrared images left and the average body surface temperature right of mice following 6. f Glucose tolerance test left and AUC right. g Insulin tolerance test left and AUC right. h iWAT weight. Lean mass was applied for normalization. l Averaged energy expenditure tested at day time and night time. m Immunoblots of total iWAT protein lysates left and quantification of protein levels right. Experiments in b — e , m were repeated independently three times, and results in f — l were repeated independently twice with similar results. To further explore the therapeutic potential of Musclin blockage for metabolic diseases, we next attempted to inactivate Musclin in the circulation using a rabbit anti-Musclin neutralizing antibody Musclin Ab and examined the subsequent effects of this Musclin inactivation on the thermogenic metabolism in mice Fig. As shown in Fig. We then utilized this neutralizing antibody to treat both chow and HFD-fed mice through intraperitoneal injection using saline as a control. Interestingly, a relatively low dose of Musclin Ab 0. Accordingly, the relative levels of p-PKA substrates and p-HSL were elevated in iWAT by Musclin Ab treatment Fig. Furthermore, Musclin Ab treatment effectively promoted oxygen consumption Fig. Together, these findings suggest that the inactivation of Musclin is a practical approach to activating beige fat thermogenesis and improving systemic energy homeostasis in mice, and thereby holds therapeutic potential for obesity and other associated metabolic diseases in humans. WT male mice were treated with saline or Musclin Antibody Musclin Ab. a Schematic of in vitro validation of Musclin antibody and in vivo Musclin neutralization assay. c Body weight gain. d Weight of indicated tissues. f Immunoblots of total lysates from iWAT left , and quantification of protein levels right. g—i Chow diet-fed mice were treated with saline or Musclin Ab beginning at 2. i Average energy expenditure at day time or night time. j Core body temperature of HFD-fed mice during acute cold exposure at 4°C. HFD feeding began at 2. k Blood glucose levels. HFD feeding began in mice at 3. l Glucose tolerance test left and the AUC right. Experiments in b , f , j , k were repeated independently three times, and results in c — e , g — i , l were repeated independently twice with similar results. Skeletal muscle, the central organ for postprandial glucose disposal, is critical for controlling the glucose metabolism and energy homeostasis of the whole-body 1 , 2 , 3 , 4. Beyond nutrient and fuel metabolism, skeletal muscle has also emerged as an endocrine organ to maintain systemic energy balance and disease progression through myokine-mediated muscle-tissue crosstalk 5 , 6. Brown and beige fat thermogenesis and function have attracted considerable attention over the past decade due to their energy dissipating features and therapeutic potential for obesity-associated metabolic disorders. Study of their related regulatory mechanisms have led to the identification of several local and systemic secreted factors that activate brown and beige fat energy expenditure and improve systemic energy homeostasis These include several myokines such as Irisin 33 , Meteorin-like Metrnl 34 , and β-aminoisobutyric acid BAIBA However, whether skeletal muscle and adipose tissue are horizontally interconnected in response to environmental temperature challenges to precisely control systemic thermogenesis and metabolic homeostasis remains unclear. In the present work, we identified Musclin as a temperature sensitive myokine that can directly bind to beige adipocyte and inhibit its thermogenesis through antagonizing Tfr1-PKA mediated thermogenic induction. Muscle expression of Musclin is notably increased in obese and diabetic mice and humans, leading to a corresponding elevation of Musclin levels in the circulation. Under chow diet-fed conditions, Musclin is critical for the regulation of murine body temperature and glucose homeostasis in response to cold exposure. Moreover, muscle-specific transgenic or AAV-mediated elevation of circulating Musclin levels inhibits beige fat metabolism and exacerbates HFD-induced obesity, insulin resistance and glucose intolerance. More importantly, inhibition of Musclin action by genetic ablation or neutralizing antibody treatment promotes beige fat thermogenesis, energy expenditure, and ameliorates HFD-induced metabolic defects. In this way, we have revealed temperature-regulated myokine Musclin as a potential therapeutic target for treating obesity and associated metabolic diseases. Musclin has also been termed Osteocrin since it was initially found to be expressed in osteoblasts and young osteocytes that modulates osteoblast function, developmental bone growth 23 , 36 , and physiological load-induced bone formation Musclin was subsequently also identified as a novel secretory factor preferentially expressed in skeletal muscle in adult rodents However, as a muscle-enriched secretory factor, the role and underlying mechanisms of Musclin in regulating systemic thermogenesis and metabolic health remain largely unknown. Here we uncovered Musclin as a critical negative regulator of beige fat thermogenesis and energy expenditure, linking muscle bioenergetic and nutrient-sensing functions to the control of systemic metabolic homeostasis under both physiological cold exposure and pathophysiological diet-induced obesity conditions through muscle-beige fat crosstalk. There is mounting evidence suggesting the influences exerted by different bone-derived molecules in systemic metabolism It is possible that Musclin from bone may also play a role in the regulation of systemic energy metabolism in adult mice. Consistent with the previous study 24 , the tibia, a long bone in the hindlimb, displayed a markedly lower expression of Musclin gene than that in skeletal muscle in adult mice. In addition, we also examined the Musclin mRNA levels in both skeletal muscle and long bone from Musclin-MKO and control mice, and showed that muscle-specific knockout of Musclin does not influence its expression in bone. These data indicate that skeletal muscle-derived Musclin plays a pivotal role in the regulation of adipose tissue thermogenesis and systemic energy homeostasis. Nonetheless, we also noticed that the plasma concentration of Musclin was partially decreased in Musclin-MKO mice, suggesting that other tissues may also contribute to the circulating levels of Musclin. The roles of Musclin secreted from these tissues in the regulation of tissue homeostasis and energy metabolism remain to be explored in future studies. Interestingly, Musclin can be repurposed in primates through the evolutionary acquisition of DNA regulatory elements to regulate neuronal structure and function features that are unique to primates 39 , suggesting a species-specific function of Musclin. More recently, Musclin was shown to function as an exercise-responsive myokine 40 , to attenuate the pathogenesis and progression of several cardiovascular diseases including heart failure during pathological overload 41 , hypertension 42 , cardiac remodeling and congestive heart failure after myocardial infarction 43 , and chronic doxorubicin-induced cardiotoxicity 44 in animal models. In addition, low circulating Musclin levels are associated with adverse prognosis of patients undergoing transcatheter aortic valve implantation TAVI 45 , and potential atrial fibrillation in non-diabetic patients Taken together, these findings demonstrate the tissue-specific and context-dependent roles of Musclin under physiological and pathological conditions. Using SEAP binding assay, we demonstrated that Musclin mainly binds to adipose tissues, especially subcutaneous iWAT, rather than other metabolic tissues, such as skeletal muscle and liver. Further studies revealed that elevated circulating Musclin selectively acts on iWAT to suppress thermogenic gene expression programs, resulting in impairment of beige fat thermogenesis, lower energy expenditure, and augmentation of HFD-induced obesity and metabolic dysfunction. Consistently, both MCK-Musclin and Musclin-MKO mice used in this study exhibited no detectable differences in muscle morphology, exercise performance, or energy metabolism-related gene expression profiles compared to their respective controls. However, a previous study demonstrated that Musclin whole-body knockout mice displayed impaired oxidative metabolism in skeletal muscle and reduced exercise tolerance compared to controls following five consecutive days of exercise training Three reasons might contribute to this discrepancy. Firstly, to exclude the potential confounding effects of Musclin on muscle development and bone growth 23 , 36 , we generated mice carrying muscle-specific transgenic expression and knockout of Musclin using MCK promoter 47 and MLC-Cre 48 , respectively. This lies in contrast to the Musclin whole-body knockout mice utilized in their study. Secondly, different running protocols and testing conditions were used in the two studies. Whilst our evaluation incorporated all the muscle-related parameters in sedentary mice, in their research mice were subjected to 5 consecutive days of exercise training prior to assessment They actually found that differences in endurance running capacity and markers of mitochondrial biogenesis were much less evident in sedentary WT and Musclin-KO mice than those in exercise-trained WT and Musclin-KO mice. Thirdly, different receptors and associated downstream signal pathways might be another reason for the tissue-specific effects of Musclin on mitochondrial biogenesis in skeletal muscle and adipose tissue. However, in this study, we identified Tfr1 as a receptor for Musclin in adipocytes, and revealed that Tfr1-mediated cAMP signaling plays a pivotal role in the regulation of thermogenic metabolism in subcutaneous white adipose tissue. However, recent studies have demonstrated distinguishing features and mechanisms underlying the developmental origins and functional regulation of these two cell types 14 , This study demonstrated that Musclin selectively repressed gene expression networks related to lipid and glucose metabolism in the subcutaneous beige fat depot, resulting in the impairment of thermogenesis and augmentation of HFD-induced obesity and metabolic dysfunction. This highlighted a distinct regulatory mechanism related to beige fat thermogenic metabolism beiging with a unique role in the pathogenesis of obesity and its associated metabolic dysfunction. Receptors are generally indispensable for mediating the vital role of secreted proteins in the crosstalk among tissues. Musclin has been shown to function as a ligand to Npr3, competing with natriuretic peptides NPs , to amplify the NP signaling 36 , As such, it is possible that Musclin may elicit its effects on adipocyte thermogenesis and metabolism through regulating the circulation levels of NPs and the downstream cGMP levels, a key player in mediating NP-associated responses Consistently, a recent study by Szaroszyk M et al. Conversely, plasma CNP levels were lower in muscle-specific Musclin deficient mice versus control mice after TAC surgery However, plasma ANP levels were either trending lower or higher in Musclin overexpressing mice after myocardial infarction 43 or TAC surgery 41 , respectively. Moreover, it has been reported that plasma ANP levels were trending lower, but did not reach statistical significance, in Musclin whole-body KO mice compared to WT controls following exercise training These results revealed an overall modest effect of Musclin overexpression or deficiency on plasma ANP levels in mice. Consistent with these findings, we observed that plasma ANP levels remained largely unchanged in MCK-Musclin mice compared to control mice in response to cold exposure or HFD feeding Supplementary Fig. Notably, plasma CNP levels were significantly increased in MCK-Musclin mice compared to controls under either cold exposure or HFD feeding conditions Supplementary Fig. The cGMP, generated by the guanylyl cyclase domain of NPRA receptor for ANP and BNP and NPRB receptor for CNP , is a key mediator of the NP-associated biological responses It has been reported that Musclin could enhance cardiomyocyte cAMP generation through cGMP-mediated inhibition of cAMP-degrading phosphodiesterase 3 PDE3 Previous studies showed that both ANP and CNP could increase the intracellular cGMP levels in adipocytes 51 , Consistently, we also demonstrated that both ANP Supplementary Fig. However, co-treatment with Musclin exhibited no obvious effect on intracellular cGMP levels in response to increasing amounts of ANP or CNP Supplementary Fig. Interestingly, using the proximity-dependent biotin identification BioID assay and mass spectrometry analysis, we identified Tfr1 as the membrane receptor for Musclin in mediating its inhibitory effect on the thermogenic metabolism in beige adipocytes. Tfr1, as a member of the transferrin receptor family, is known to play an important role in cellular iron homeostasis through the endocytosis of transferrin-bound iron 53 , One recent study has demonstrated that Tfr1-mediated control of cellular iron levels is important for regulating white adipose tissue homeostasis Intriguingly, accumulating evidence indicates that other iron-independent mechanisms might also be involved in the regulation of biological function by Tfr1 Accordingly, Tfr1 was uncovered to play an essential role in regulating brown and beige fat development and thermogenesis via both iron-dependent and -independent mechanisms 29 , Consistent with the selectivity of Musclin on the thermogenesis of beige adipocytes, we observed that the protein levels of Tfr1 in adipocytes from iWAT were significantly higher than those from eWAT and BAT in WT mice following chronic cold exposure Supplementary Fig. However, whether iron-dependent and -independent mechanisms are involved in this process warrants further investigation. In summary, this work identifies the myokine Musclin as a critical negative regulator of beige fat thermogenesis that acts in concert with other thermogenesis activators to fine-tune systemic energy balance under both physiological and pathophysiological conditions. Muscle expression of Musclin is regulated by ambient temperature and is essential for maintaining body temperature upon acute and chronic cold stresses, thus indicating its physiological role in gauging systemic energy expenditure. Musclin gain-of-function inhibits beige fat thermogenesis and augments HFD-induced obesity and metabolic disorders. More importantly, blocking Musclin actions, either by genetic ablation or neutralizing antibody treatment, promotes energy expenditure and alleviates HFD induced-obesity and metabolic dysfunction. These findings highlight the therapeutic potential of Musclin inactivation for treating obesity and its associated metabolic disorders. All animal studies were performed in compliance with the Guide for the Use and Care of Laboratory Animals by the Medical Experimental Animal Care Committee of Zhejiang University. All animal studies were performed following the protocols approved by the Animal Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University Approval number: The study on human plasma was approved by the Second Affiliated Hospital of Soochow University Approval number: JD-LK Human biological samples including skeletal muscle from a total of 54 donors; 41 male and 13 female; data shown in Fig. Sex was comparable between groups, as shown in Supplementary Fig. Sex information for human sample-related results shown in Fig. Only the participants who had provided written informed consent were included in the study. All participants representing donor tissue samples were subjected to medical history inquiries before hospitalization. Blood biochemical tests were taken after overnight starvation. Subjects with other severe diseases, including malignant tumors and severe obesity-independent cardiovascular disorders, were also excluded. Moreover, to minimize the confounding effect of previous injury in the current findings, the human muscle samples were only collected from patients who have fully recovered from acute injury with confirmation of the absence of acute inflammation prior to ligament repair or reconstruction treatments. BMI is the only criterion to divide the human muscle samples into different groups. As such, we assumed that the physical activity should be similar between groups. All the patients for scWAT collection were checked for full recovery from acute injury and confirmation of the absence of inflammation before surgery. Fat samples from subjects with malignant tumors were excluded. All the tissue samples were freshly collected and immediately frozen in liquid nitrogen. Human blood samples were collected from subjects with differing BMIs, while subjects with malignant tumors, organic lesions, or having previously undergone surgery within several months to half a year were excluded. Male mice were used for all the experiments unless otherwise indicated. On rare occasions, mice in bad health conditions, such as severe fighting wounds or exhibiting sickness, according to the predefined criteria listed in the Institutional Animal Care and Use Committee IACUC protocol were excluded. All in vivo experiments were independently repeated at least twice. To generate skeletal muscle-specific Musclin transgenic MCK-Musclin mice by pronuclear microinjection, a full-length Musclin coding sequence was placed downstream of the 4. Liwei Xie Guangdong Institute of Microbiology. Adipoq-Cre JAX stock mice were generously provided by Dr. Fudi Wang Zhejiang University , and have been previously described 29 , Cre expression in Adipoq-CreERT2 mice was activated through intraperitoneal administration of tamoxifen at the dose of 0. All mouse strains used in this study were born at the expected Mendelian ratios with normal fertility. Musclin neutralizing Ab preparation was performed by ABclonal Technology Co. Through epitope prediction, the peptide sequence C-HSKKRFGIP-Nle-DRIGRNR corresponding to aa of Musclin was synthesized and used for immunizing rabbits to generate antibodies. Polyclonal antibodies against Musclin were obtained from inoculated rabbits. Antibodies were purified using affinity chromatography on columns containing the corresponding peptides. Female mice were only used for the cold exposure study shown in Fig. The mouse age and sex information for each experiment has also been included in the corresponding figure legend. ExpiF cell line was obtained from Thermo Fisher. AAV cell line was purchased from Agilent. HEKT, ExpiF, and AAV cell lines were authenticated and routinely used in our previous studies 2 , 59 , 60 , HUVEC cell line was kindly provided by Dr. Nan Xu Henan University , and has been authenticated and successfully used in their previous study AAV production and purification were performed by ChuangRui Bio Lianyungang, China. Culture media were replaced with DMEM plus 0. Cell lysates were gently transferred onto the top layer, and the remaining volume of the ultracentrifuge tube was filled with cell lysis buffer. The viral titer was determined by qPCR assay with a standard curve generated by serial dilutions of the AAV shuttle vector. Mice were granted free access to pre-chilled food and water during the whole assay. Core body temperature was monitored at indicated time points using a portable intelligent digital thermometer TH During this period, metabolic parameters including mouse body temperature, body weight, blood glucose, as well as plasma TG and NEFA levels were monitored. Body surface temperature was measured using a thermal imaging camera FLIR Systems, Tsc InfraRed Camera. Mice were anesthetized with isoflurane and quietly laid on a whiteboard with their back up, followed by image capturing with the camera anchored at the same height for all mice in the same batch. FLIR Tools 5. Food and water were freely accessible to mice. O 2 consumption, CO 2 production, energy expenditure, total locomotor activity, and food intake were monitored under both normal conditions and under adrenergic stimulation. The metabolic parameters for each mouse were measured two days before CL injection and were continued to be monitored for one more day after CL injection. Mouse running performance was assessed using the treadmill running system from Columbus Instruments. The inclination angle was level. Total running distance and running time were recorded at the point when the mice reached exhaustion. After full differentiation, myotubes were subject to RNA isolation and gene expression analysis. ExpiF cells were transfected with pcDNA3. The XF96 microplate was then loaded into a Seahorse XFe96 analyzer for equilibration and determination of the basal respiration rate. OCRs were recorded and the ATP production, maximal respiration, and the spare respiratory capacity-dependent OCRs were calculated. Cell viability was also quantified using the CCK8 kit Beyotime for normalization. The XF Cell Mito Stress test kit Agilent was used in this assay. Seahorse Wave Desktop and Controller 2. An NMR analyzer NIUMAG, QMNH was applied to measure the body fat and lean mass. An enzyme-linked immunosorbent assay ELISA kit from Crystal Chem was used to measure plasma insulin concentration. For the detection of Musclin levels in human plasma, a Human Musclin ELISA kit CUSABIO, CSB-Eh was applied. For the detection of ANP levels in mouse plasma, a mouse ANP ELISA kit Elabscience, E-EL-Mc was used. For the detection of CNP levels in mouse plasma, a Mouse CNP ELISA kit Beijing Sino-UK Institute of Biological Technology, HY-NE was applied. Mouse tissues including liver, skeletal muscle, BAT, eWAT, and iWAT were dissected, fixed in formalin, followed by embedding in paraffin and cutting for tissue sections at μm for liver, skeletal muscle, and BAT, or μm for eWAT and iWAT. Tissue sections were then stained with hematoxylin and eosin, and subjected to image acquisition using NIS Elements F 4. The cell size of iWAT was quantified using Image J 1. The SEAP-binding assay was performed as described Firstly, the vectors expressing SEAP or SEAP-Musclin fusion protein were transiently transfected into HEKT cells. The sections were washed with PBS containing 0. CellSens Standard Olympus was used for image acquisition and data collection. Total RNA from WAT was isolated using a commercially available kit TIANGEN Biotech, DP Total RNA from other tissues including skeletal muscle, heart, brain, small intestine, liver, BAT, bone, and cultured cells was isolated following the standard method using TRIzol. RNA was then reverse transcribed using HiScript II Q RT SuperMix Vazyme, R , followed by qPCR analysis using SYBR Green Roche. The qPCR primers used are listed in Supplementary Data. Sequencing libraries were constructed from total RNA using SMART-RNAseq Library Prep Kit Hangzhou KaiTai, AT In brief, mRNA was isolated from total RNA with Sera-Mag Magnetic Olido dT particles, and then chemically fragmented. And the cDNA libraries were subsequently amplified using the KAPA high-fidelity DNA polymer. Quality of the libraries was validated by the Bioanalyzer Agilent Technologies. Subsequently, high-throughput sequencing was performed using a NovaSeq Illumina. Raw reads were filtered with fastp V0. Filtered data were then aligned with HISAT2 V2. p13 for human data. The FPKM fragments per kilobase of exon per million fragments mapped was calculated using StringTie V2. For iWAT from cold-acclimated and room temperature-housed control WT mice, RNA sequencing was performed in Majorbio Bio-pharm Biotechnology Co. Shanghai, China. The library was prepared using TruSeqTM RNA Sample Prep Kit Illumina. Shortly, mRNA was isolated by oligo dT beads and then fragmented. After quantified by TBS, paired-end RNA sequencing was performed using the NovaSeq Illumina. The raw reads were processed similarly and the TPM transcripts per million reads values were used to determine gene expression levels. For human scWAT, RNA sequencing was performed in BGI Shenzhen, China. Similarly, mRNA was purified using oligo dT -attached magnetic beads and was then fragmented. cDNA was generated by random hexamer-primed reverse transcription, and end-repaired through incubation with a-Tailing Mix and RNA Index Adapters. The obtained cDNA fragments were amplified using PCR followed by purification with Ampure XP Beads, which was validated on the Bioanalyzer Agilent Technologies. Distinctively, further heat-denaturation and circularization by the splint oligo sequence of the PCR products from previous step were needed to get the final library. Pair end base reads were generated for analysis. The sequencing data was filtered with SOAPnuke V1. p12 with HISAT2 V2. Expression level of gene FPKM value was calculated by RSEM V1. For all these sequencing data, differential expression analysis was performed using the Deseq2 v. Gene Ontology GO and pathway grouping and enrichment studies were performed by clusterProfiler V3. Html Results were visualized by ggplot2 V3. html 70 and pheatmap V1. Protein lysates from skeletal muscles and adipose tissues, or whole-cell lysates from cultured cells were quantified using BCA protein assay Beyotime. The final results were visualized with chemiluminescence ECL western blotting substrates. A proximity-dependent biotin identification BioID assay was used to identify the receptors for Musclin on the plasma membrane of adipocytes. The agarose beads were pelleted by centrifugation and then subjected to five times washing with washing buffer, followed by protein denaturation, SDS-PAGE gel electrophoresis, and Coomassie blue staining or silver staining Pierce Silver Stain Kit , Thermo Fisher. The gel fragments in pulldown samples containing differential protein bands as compared to control were collected for electrospray ionization tandem M. analysis on a Thermo Finnigan LTQ Orbitrap Instrument Proteome Discoverer version 1. The mass spectrometry proteomic data have been deposited to the ProteomeXchange Consortium via the PRIDE 74 partner repository with the dataset identifier PXD HEKT cells were transiently transfected with plasmids expressing control or Musclin. Finally, cells and culture media were harvested separately. Cells were subjected to total protein lysate preparation. The obtained protein pellet was washed in cold acetone, air-dried, and finally resuspended in SDS-containing lysis buffer. The input and IP samples were subjected to immunoblotting using antibodies against Musclin Abcam , Flag Sigma, A, M2 , or HSL Cell signaling, s. Cell proteins were quantified using a BCA protein assay kit Beyotime for normalization. Tissue weight was used for normalization. A standard chromogen method was used to measure the tissue non-heme iron described as previously described All statistical analyses were performed using GraphPad Prism 9 software. n values represent biological replicates for cell experiments, or mouse number for in vivo animal studies, or human subject number unless otherwise indicated. Specific details for the n value are noted in each figure legend. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. RNA-Seq data of iWAT from cold-acclimated mice and their controls are available in SRA database under accession code: PRJNA The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE 74 partner repository with the dataset identifier PXD , which can be freely accessed. The images representing human participants, mouse models, skeletal muscle, myotube, adipose tissue, RNA-Sequencing, qPCR analysis, culture dish, and adipocyte shown in Figs. Source data are provided with this paper. Meng, Z. et al. Glucose sensing by skeletal myocytes couples nutrient signaling to systemic homeostasis. Cell 66 , — e Article CAS PubMed PubMed Central Google Scholar. Baf60c drives glycolytic metabolism in the muscle and improves systemic glucose homeostasis through Deptor-mediated Akt activation. Diabetes 63 , — Uncoupling exercise bioenergetics from systemic metabolic homeostasis by conditional inactivation of Baf60 in skeletal muscle. Diabetes 67 , 85—97 Article CAS PubMed Google Scholar. Pedersen, B. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Febbraio, M. Who would have thought—myokines two decades on. Article PubMed Google Scholar. Whitham, M. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. Giudice, J. Muscle as a paracrine and endocrine organ. Article CAS Google Scholar. Priest, C. Inter-organ cross-talk in metabolic syndrome. Ishibashi, J. Beige can be slimming. Science , — Petrovic, N. Chronic peroxisome proliferator-activated receptor gamma PPARgamma activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. Rosen, E. What we talk about when we talk about fat. Cell , 20—44 Ikeda, K. The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Wu, J. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell , — Matthew, H. Brown and beige fat: development, function and therapeutic potential. Article Google Scholar. Townsend, K. Brown fat fuel utilization and thermogenesis. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Med 23 , — Kaisanlahti, A. Browning of white fat: agents and implications for beige adipose tissue to type 2 diabetes. Biochem 75 , 1—10 Carobbio, S. Brown and beige fat: From molecules to physiology and pathophysiology. Acta Mol. Cell Biol. lipids , 37—50 Blüher, M. Obesity: global epidemiology and pathogenesis. Wang, Q. The hepatokine Tsukushi gates energy expenditure via brown fat sympathetic innervation. Article PubMed PubMed Central Google Scholar. Xiong, X. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Cell 75 , — Thomas, G. Osteocrin, a novel bone-specific secreted protein that modulates the osteoblast phenotype. Nishizawa, H. Musclin, a novel skeletal muscle-derived secretory factor. Kazak, L. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Roux, K. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. Li, J. CAS PubMed Google Scholar. Tsai, T. Sim, J. P2X1 and P2X4 receptor currents in mouse macrophages. Shen, W. Interaction of rat hormone-sensitive lipase with adipocytelipid-binding protein. Natl Acad. USA 96 , — Article ADS CAS PubMed PubMed Central Google Scholar. Bostrom, P. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature , — Article ADS PubMed PubMed Central Google Scholar. Rao, R. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Roberts, L. β-Aminoisobutyric acid induces browning of white fat and hepatic β -oxidation and is inversely correlated with cardiometabolic risk factors. Moffatt, P. Osteocrin is a specific ligand of the natriuretic peptide clearance receptor that modulates bone growth. Watanabe-Takano, H. Mechanical load regulates bone growth via periosteal Osteocrin. Cell Rep. Zhou, R. Endocrine role of bone in the regulation of energy metabolism. Bone Res. Ataman, B. Evolution of Osteocrin as an activity-regulated factor in the primate brain. Subbotina, E. Musclin is an activity-stimulated myokine that enhances physical endurance. USA , — Szaroszyk, M. Skeletal muscle derived Musclin protects the heart during pathological overload. Li, Y. Role of musclin in the pathogenesis of hypertension in rat. PLoS One 8 , e Miyazaki, T. A new secretory peptide of natriuretic peptide family, osteocrin, suppresses the progression of congestive heart failure after myocardial infarction. Hu, C. Osteocrin attenuates inflammation, oxidative stress, apoptosis, and cardiac dysfunction in doxorubicin-induced cardiotoxicity. Kattih, B. Low circulating musclin is associated with adverse prognosis in patients undergoing transcatheter aortic valve implantation at low-intermediate risk. Heart Assoc. Zhong, Y. Decreased plasma musclin levels are associated with potential atrial fibrillation in non-diabetic patients. Sternberg, E. Identification of upstream and intragenic regulatory elements that confer cell-type-restricted and differentiation-specific expression on the muscle creatine kinase gene. CAS PubMed PubMed Central Google Scholar. |
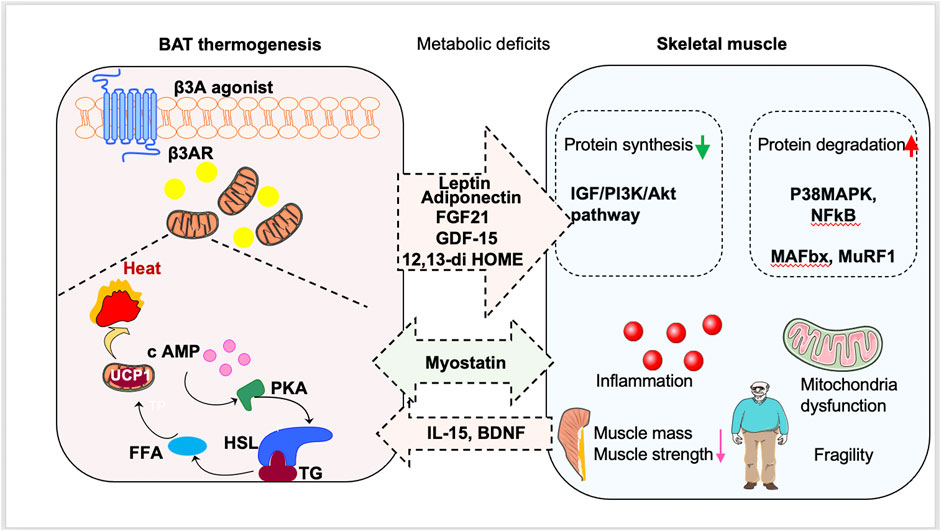
| Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism | Differential expression analysis of RNA-Seq data was performed using Deseq2 package P -value by Wald test in b — d. Metabolic changes associated with sustained hr shivering thermogenesis in the newborn pig. All in vivo experiments were independently repeated at least twice. Behaviour and physiology of Svalbard barnacle geese Branta leucopsis during their autumn migration. Febbraio, M. |
| Skeletal Muscle Thermogenesis and Its Role in Whole Body Energy Metabolism | In addition to UCP3-associated uncoupling and thermogenesis, recent data suggest that SERCA-driven beige cell thermogenesis also occurs in pigs. Indeed, the work by Ikeda et al. Ikeda et al. Retroviral expression of PRDM in subcutaneous porcine adipocytes increases the expression of beige-cell-specific markers including CIDEA and TMEM26 Ikeda et al. Furthermore, decreased SERCA2b expression reduced basal and noradrenaline-induced oxygen consumption and extracellular acidification rates in isolated pig adipocytes Ikeda et al. Thus, it is now clear that adipose tissue thermogenesis and the associated energy expenditure are not solely mediated via UCP1 and mitochondrial uncoupling, but in fact, a number of cellular pathways, across both adipose tissue and skeletal muscle, act in concert to determine total thermogenic potential. In lambs, the expression of UCP1 is maximal in perirenal adipose tissue on the first postnatal day, rapidly declining with the expansion of WAT Symonds , Pope et al. Mapping of UCP1 mRNA in lambs shows abundant expression in sternal and retroperitoneal adipose depots compared to omental fat, which is a predominantly WAT depot Symonds et al. Indeed, adult sheep retain UCP1 expression in both sternal and retroperitoneal fat and this coincides with post-prandial heat production, albeit this response is greater in the sternal fat depot Henry et al. This coincides with the expression of UCP1 protein, where UCP1-positive brown-like adipocytes were only detectable in sternal adipose tissue of adult ewes Henry et al. Data logger temperature probes have been employed to measure longitudinal heat production in multiple tissues to index thermogenic output in sheep. Sheep are a grazing species and therefore do not display typical meal-associated excursions such as changes in ghrelin secretion. Despite this, temporal food restriction in sheep entrains a pre-prandial rise in ghrelin Sugino et al. Furthermore, post-prandial thermogenesis in both skeletal muscle and retroperitoneal adipose depots is markedly enhanced by intracerebroventricular infusion of leptin Henry et al. Thus, in spite of relatively low levels of UCP1 in adult sheep, skeletal muscle and specific adipose depots retain thermogenic capacity. Over recent years, we have utilised the sheep to dissect the differential roles of adipose tissue and skeletal muscle thermogenesis in the long-term control of body weight, which is discussed in detail in the following section. Similar to other species, ovine body weight can be readily manipulated through dietary management Henry et al. Sheep are ruminants and thus body weight is increased through feeding a high-energy diet enriched in lupin grain and oats. Diet-induced obesity, however, is not associated with any change in heat production in adipose tissues or skeletal muscle of sheep Henry et al. On the other hand, long-term food restriction and low body weight are associated with a homeostatic decrease in thermogenesis in sternal and retroperitoneal adipose tissue and skeletal muscle Henry et al. Importantly, similar to humans, the reduction in thermogenesis caused by food restriction and low body weight is still evident at one year post-weight loss, which suggests that homeostatic changes in thermogenesis contribute to impaired weight loss and increased long-term weight regain Henry et al. Effect of chronic food restriction and weight loss on adaptive thermogenesis in ewes. Tissue temperature recordings show that caloric restriction and low body weight cause a homeostatic decrease in night time thermogenesis in ovariectomised ewes. This metabolic adaptation occurs in both sternal adipose tissue adipose tissue enriched in uncoupling protein 1 and skeletal muscle and to a lesser extent in retroperitoneal adipose tissue. The reduction in thermogenesis is associated with increased expression of neuropeptide Y NPY in the arcuate nucleus and melanin-concentrating hormone MCH in the lateral hypothalamus. The homeostatic reduction in thermogenesis is coordinated by the hypothalamus. Long-term weight loss in ovariectomised ewes increases the expression of the orexigenic neuropeptides NPY in the arcuate nucleus and melanin-concentrating hormone MCH in the lateral hypothalamus LH to increase hunger and reduce energy expenditure Henry et al. Regarding the anorexigenic melanocortin pathway, the effect of low body weight on the expression of POMC is controversial with data showing a decrease Backholer et al. This is not surprising since POMC is the precursor to multiple neuropeptides, only one of which includes aMSH and the ultimate end product is dependent on post-translational processing Mountjoy On the other hand, increased Agrp and Npy expression and reduced Pomc mRNA have been observed in rodents Bi et al. Thus, weight-loss-induced changes in hypothalamic gene expression are likely to reduce thermogenesis, whilst causing a concurrent increase in hunger drive. This represents a homeostatic mechanism to protect against weight loss and promote weight regain in calorie-restricted individuals. Animals were originally selected for innate differences in adiposity by measuring back fat thickness and two lines were created via selective breeding strategies. A key feature of the genetically lean and obese sheep is an inherent difference in the growth hormone GH axis, where lean animals have increased mean GH concentration in plasma and an associated increase in pituitary gland weight Francis et al. The increase in pituitary gland weight is primarily due to a greater number of cells in the lean animals Francis et al. Furthermore, expression of GH and the GH secretagogue receptor GHSR is greater in genetically lean sheep, indicating differential responses to ghrelin, an agonist of the GHSR French et al. This suggests that innate differences in the set-point of the GH axis may underpin differences in adiposity in the genetically lean and obese sheep; however, this is only one aspect that could contribute to this phenotype. Interestingly, food intake is similar in genetically lean and obese sheep as is the expression of POMC, Leptin Receptor and NPY in the arcuate nucleus. On the other hand, lean animals have elevated post-prandial thermogenesis in retroperitoneal adipose tissue and this coincides with increased expression of UCP1 in this tissue Henry et al. The divergence in thermogenesis is specific to adipose tissue since post-prandial thermogenesis is similar in genetically lean and obese animals Henry et al. Despite similar expression of appetite-regulating peptides in the arcuate nucleus of the hypothalamus, genetically lean sheep have increased expression of MCH and pre-pro-orexin ORX in the LH compared to obese animals Anukulkitch et al. While both neuropeptides are considered orexigenic Shimada et al. Deletion of MCH in mice results in hypophagia and a lean phenotype Shimada et al. Orexin is critical in the embryonic development of BAT in mice Sellayah et al. Thus, increased expression of ORX in the LH of lean sheep may be an important physiological determinant of increased thermogenesis in retroperitoneal fat and the associated changes in adiposity. It is widely recognised that there is marked variation in the glucocorticoid response to stress or activation of the hypothalamo-pituitary adrenal HPA axis Cockrem , Walker et al. The activity of the HPA axis in response to stress is impacted on by age Sapolsky et al. Nonetheless, in any given population individuals can be characterised as either high HR or low LR glucocorticoid responders Epel et al. It is important to note that female LR and HR sheep have similar basal plasma cortisol concentration and divergence in glucocorticoid secretion only occurs in response to ACTH or stress Lee et al. Previous studies have suggested that obesity itself causes perturbation of the HPA axis with impaired glucocorticoid-negative feedback Jessop et al. Furthermore, cortisol directly impacts on metabolic function; however, this will not be addressed in the current review. Initial studies in rams show that high cortisol response to adrenocorticotropin ACTH is associated with lower feed-conversion efficiency Knott et al. Furthermore, in rams, adiposity is correlated to cortisol responses to ACTH Knott et al. More recent work shows that identification of high HR and low LR cortisol responders in female sheep can predict altered propensity to gain weight when exposed to a high-energy diet, where HR gain more adipose tissue than LR Lee et al. Thus, at least in female sheep, data suggest that cortisol responses can be used as a physiological marker that predicts propensity to become obese. Previous studies in women suggest that HR eat more after a stressful episode than LR Epel et al. Furthermore, HR individuals display preference for foods of high fat and sugar in response to psychological stress Tomiyama et al. Similarly, in ewes, baseline food intake is similar in LR and HR, but HR eat more following either psychosocial barking dog or immune lipopolysaccharide exposure stressors Lee et al. In addition to altered food intake, HR ewes have reduced thermogenesis in skeletal muscle only; in response to meal feeding, post-prandial thermogenesis in skeletal muscle is greater in LR than in HR Lee et al. This again exemplifies divergence in the control of adipose tissue and skeletal muscle thermogenesis Fig. Schematic depiction of the altered metabolic phenotype in animals selected for either high or low cortisol responsiveness. Sheep are characterised as either high HR or low LR cortisol responders when given a standardised dose of adrenocorticotropic hormone. Animals characterized as HR have increased propensity to become obese, which is associated with perturbed control of food intake and reduced energy expenditure. Post-prandial thermogenesis in skeletal muscle is decreased in HR compared to LR ewes. Furthermore, food intake in response to stress is greater in HR than in LR and the former are resistant to the satiety effect of alpha-melanocyte stimulating hormone aMSH. High-cortisol-responding animals have reduced expression of the melanocortin 4 receptor MC4R in the paraventricular nucleus of the hypothalamus PVN. We propose that the decreased levels of MC4R underpin the altered metabolic phenotype and increased propensity to become obese when compared to LR. For example, at baseline in the non-stressed resting state, HR individuals show an overall upregulation of the HPA axis, with increased expression of CRF and arginine vasopressin, but reduced expression of oxytocin in the PVN Hewagalamulage et al. In addition to altered expression of genes within the HPA axis, a key neuroendocrine feature of the LR and HR animals is altered expression of the MC3R and MC4R in the PVN Fig. Reduced MC4R expression coincides with the development of melanocortin resistance. Central infusion of leptin reduces food intake in both LR and HR animals, but intracerebroventricular infusion of aMSH reduces food intake in LR only. Thus, reduced MC4R expression appears to be central to the metabolic phenotype of HR that confers increased propensity to become obese in HR individuals Fig. Interestingly, gene expression of NPY , AgRP and POMC in the arcuate nucleus is equivalent in LR and HR Hewagalamulage et al. Hence, differences in the control of food intake and thermogenesis are most likely manifest at the level of the melanocortin receptor. Indeed, previous work in sheep has shown the MC4R to be central in mediating the reduction in food intake caused by immune challenge Sartin et al. Furthermore, in rodents, direct injection of the melanocortin agonist melanotan II into the ventromedial nucleus of the hypothalamus increases skeletal muscle thermogenesis Gavini et al. We propose that reduced expression of the MC4R in HR animals underpins the metabolic phenotype wherein food intake is relatively increased in response to stress and reduced post-prandial thermogenesis in skeletal muscle is associated with propensity to become obese. Historically, thermogenesis was considered to primarily occur in brown adipocytes and was solely driven by UCP1. It is now recognised that beige adipocytes and skeletal muscle also contribute to total thermogenic capacity and that thermogenesis is differentially regulated in these tissues. Indeed, in beige adipocytes, thermogenesis occurs via three distinct mechanisms, with these being UCP1-driven mitochondrial uncoupling, futile creatine cycling and futile calcium cycling. On the other hand, in skeletal muscle, thermogenesis is associated with UCP3 and futile calcium cycling. Unlike rodents, large mammals including sheep and pigs do not contain a defined or circumscribed brown fat depot but have dispersed brown adipocytes within traditionally white fat depots. Large animals have provided invaluable insight into alternative mechanisms of thermogenesis. The sheep has been particularly useful in delineating the differential role of adipose tissue and skeletal muscle in the control of body weight. Furthermore, sheep models have allowed characterisation of the neuroendocrine pathways that may contribute to altered thermogenesis. We have shown that in sheep, both skeletal muscle and BAT differentially contribute to thermogenesis and therefore total energy expenditure. Changes in thermogenesis, however, do not exclusively associate with altered gene expression at the level of the arcuate nucleus. Indeed, decreased MC4R expression in HR animals and reduced orexin expression in the genetically obese animals coincide with altered thermogenic output. This review highlights the importance of the use of large animal models to ascertain the contribution and control of thermogenesis in multiple tissues and the relative role in the regulation of body weight. The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review. This work was supported by Australian Research Council grant number DP and National Health and Medical Research Council grant number APP Animal Science 63 — Journal of Pathology and Bacteriology 91 — Obesity Reviews 19 — Molecular Metabolism 5 — Neuroendocrinology 91 — Biochimica et Biophysica Acta — Astrup A Thermogenesis in human brown adipose tissue and skeletal muscle induced by sympathomimetic stimulation. Acta Endocrinologica Supplement S9 — S American Journal of Physiology E — E Science — Neuroendocrinology 91 27 — Journal of Biological Chemistry — Bal NC , Maurya SK , Sopariwala DH , Sahoo SK , Gupta SC , Shaikh SA , Pant M , Rowland LA , Bombardier E , Goonasekera SA , et al. Nature Medicine 18 — Journal of Neuroendocrinology 29 e Balthasar N , Dalgaard LT , Lee CE , Yu J , Funahashi H , Williams T , Ferreira M , Tang V , McGovern RA , Kenny CD , et al. Cell — American Journal of Physiology R — R Banks WA Characteristics of compounds that cross the blood-brain barrier. BMC Neurology 9 S3 — S3. American Journal of Physiology: Endocrinology and Metabolism E — E Disease Models and Mechanisms 9 — Cellular and Molecular Life Sciences 73 — PLoS Genetics 2 e Cell Metabolism 25 e — Lancet — Circulation Research — American Journal of Physiology: Regulatory, Integrative and Comparative Physiology R — R International Journal of Obesity and Related Metabolic Disorders 17 Supplement 3 S78 — S Blondin DP , Daoud A , Taylor T , Tingelstad HC , Bezaire V , Richard D , Carpentier AC , Taylor AW , Harper ME , Aguer C , et al. Journal of Physiology — International Journal of Obesity 37 — PLoS ONE 11 e Progress in Neuro-Psychopharmacology and Biological Psychiatry 35 — Metabolism 64 — Physiology Reviews 84 — Carey AL , Pajtak R , Formosa MF , Van Every B , Bertovic DA , Anderson MJ , Eikelis N , Lambert GW , Kalff V , Duffy SJ , et al. Diabetologia 58 — Endocrinology — Cinti S The adipose organ: morphological perspectives of adipose tissues. Proceedings of the Nutrition Society 60 — Claret M , Smith MA , Batterham RL , Selman C , Choudhury AI , Fryer LG , Clements M , Al-Qassab H , Heffron H , Xu AW , et al. Journal of Clinical Investigation — Clement K , Vaisse C , Lahlou N , Cabrol S , Pelloux V , Cassuto D , Gourmelen M , Dina C , Chambaz J , Lacorte JM , et al. Nature — Cockrem JF Individual variation in glucocorticoid stress responses in animals. General and Comparative Endocrinology 45 — Diabetes 64 — Coppola A , Liu Z , Andrews Z , Paradis E , Roy M-C , Friedman JM , Ricquier D , Richard D , Horvath TL , Gao X-B , et al. Cell Metabolism 5 21 — Journal of Cell Science — Neuron 24 — Clinical Science 69 — Cypess AM , Lehman S , Williams G , Tal I , Rodman D , Goldfine AB , Kuo FC , Palmer EL , Tseng Y-H , Doria A , et al. New England Journal of Medicine — Cypess AM , Weiner LS , Roberts-Toler C , Elia EF , Kessler SH , Kahn PA , English J , Chatman K , Trauger SA , Doria A , et al. Cell Metabolism 21 33 — Nature Medicine 19 — Dalgaard K , Landgraf K , Heyne S , Lempradl A , Longinotto J , Gossens K , Ruf M , Orthofer M , Strogantsev R , Selvaraj M , et al. Moreover, it has been reported that plasma ANP levels were trending lower, but did not reach statistical significance, in Musclin whole-body KO mice compared to WT controls following exercise training These results revealed an overall modest effect of Musclin overexpression or deficiency on plasma ANP levels in mice. Consistent with these findings, we observed that plasma ANP levels remained largely unchanged in MCK-Musclin mice compared to control mice in response to cold exposure or HFD feeding Supplementary Fig. Notably, plasma CNP levels were significantly increased in MCK-Musclin mice compared to controls under either cold exposure or HFD feeding conditions Supplementary Fig. The cGMP, generated by the guanylyl cyclase domain of NPRA receptor for ANP and BNP and NPRB receptor for CNP , is a key mediator of the NP-associated biological responses It has been reported that Musclin could enhance cardiomyocyte cAMP generation through cGMP-mediated inhibition of cAMP-degrading phosphodiesterase 3 PDE3 Previous studies showed that both ANP and CNP could increase the intracellular cGMP levels in adipocytes 51 , Consistently, we also demonstrated that both ANP Supplementary Fig. However, co-treatment with Musclin exhibited no obvious effect on intracellular cGMP levels in response to increasing amounts of ANP or CNP Supplementary Fig. Interestingly, using the proximity-dependent biotin identification BioID assay and mass spectrometry analysis, we identified Tfr1 as the membrane receptor for Musclin in mediating its inhibitory effect on the thermogenic metabolism in beige adipocytes. Tfr1, as a member of the transferrin receptor family, is known to play an important role in cellular iron homeostasis through the endocytosis of transferrin-bound iron 53 , One recent study has demonstrated that Tfr1-mediated control of cellular iron levels is important for regulating white adipose tissue homeostasis Intriguingly, accumulating evidence indicates that other iron-independent mechanisms might also be involved in the regulation of biological function by Tfr1 Accordingly, Tfr1 was uncovered to play an essential role in regulating brown and beige fat development and thermogenesis via both iron-dependent and -independent mechanisms 29 , Consistent with the selectivity of Musclin on the thermogenesis of beige adipocytes, we observed that the protein levels of Tfr1 in adipocytes from iWAT were significantly higher than those from eWAT and BAT in WT mice following chronic cold exposure Supplementary Fig. However, whether iron-dependent and -independent mechanisms are involved in this process warrants further investigation. In summary, this work identifies the myokine Musclin as a critical negative regulator of beige fat thermogenesis that acts in concert with other thermogenesis activators to fine-tune systemic energy balance under both physiological and pathophysiological conditions. Muscle expression of Musclin is regulated by ambient temperature and is essential for maintaining body temperature upon acute and chronic cold stresses, thus indicating its physiological role in gauging systemic energy expenditure. Musclin gain-of-function inhibits beige fat thermogenesis and augments HFD-induced obesity and metabolic disorders. More importantly, blocking Musclin actions, either by genetic ablation or neutralizing antibody treatment, promotes energy expenditure and alleviates HFD induced-obesity and metabolic dysfunction. These findings highlight the therapeutic potential of Musclin inactivation for treating obesity and its associated metabolic disorders. All animal studies were performed in compliance with the Guide for the Use and Care of Laboratory Animals by the Medical Experimental Animal Care Committee of Zhejiang University. All animal studies were performed following the protocols approved by the Animal Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University Approval number: The study on human plasma was approved by the Second Affiliated Hospital of Soochow University Approval number: JD-LK Human biological samples including skeletal muscle from a total of 54 donors; 41 male and 13 female; data shown in Fig. Sex was comparable between groups, as shown in Supplementary Fig. Sex information for human sample-related results shown in Fig. Only the participants who had provided written informed consent were included in the study. All participants representing donor tissue samples were subjected to medical history inquiries before hospitalization. Blood biochemical tests were taken after overnight starvation. Subjects with other severe diseases, including malignant tumors and severe obesity-independent cardiovascular disorders, were also excluded. Moreover, to minimize the confounding effect of previous injury in the current findings, the human muscle samples were only collected from patients who have fully recovered from acute injury with confirmation of the absence of acute inflammation prior to ligament repair or reconstruction treatments. BMI is the only criterion to divide the human muscle samples into different groups. As such, we assumed that the physical activity should be similar between groups. All the patients for scWAT collection were checked for full recovery from acute injury and confirmation of the absence of inflammation before surgery. Fat samples from subjects with malignant tumors were excluded. All the tissue samples were freshly collected and immediately frozen in liquid nitrogen. Human blood samples were collected from subjects with differing BMIs, while subjects with malignant tumors, organic lesions, or having previously undergone surgery within several months to half a year were excluded. Male mice were used for all the experiments unless otherwise indicated. On rare occasions, mice in bad health conditions, such as severe fighting wounds or exhibiting sickness, according to the predefined criteria listed in the Institutional Animal Care and Use Committee IACUC protocol were excluded. All in vivo experiments were independently repeated at least twice. To generate skeletal muscle-specific Musclin transgenic MCK-Musclin mice by pronuclear microinjection, a full-length Musclin coding sequence was placed downstream of the 4. Liwei Xie Guangdong Institute of Microbiology. Adipoq-Cre JAX stock mice were generously provided by Dr. Fudi Wang Zhejiang University , and have been previously described 29 , Cre expression in Adipoq-CreERT2 mice was activated through intraperitoneal administration of tamoxifen at the dose of 0. All mouse strains used in this study were born at the expected Mendelian ratios with normal fertility. Musclin neutralizing Ab preparation was performed by ABclonal Technology Co. Through epitope prediction, the peptide sequence C-HSKKRFGIP-Nle-DRIGRNR corresponding to aa of Musclin was synthesized and used for immunizing rabbits to generate antibodies. Polyclonal antibodies against Musclin were obtained from inoculated rabbits. Antibodies were purified using affinity chromatography on columns containing the corresponding peptides. Female mice were only used for the cold exposure study shown in Fig. The mouse age and sex information for each experiment has also been included in the corresponding figure legend. ExpiF cell line was obtained from Thermo Fisher. AAV cell line was purchased from Agilent. HEKT, ExpiF, and AAV cell lines were authenticated and routinely used in our previous studies 2 , 59 , 60 , HUVEC cell line was kindly provided by Dr. Nan Xu Henan University , and has been authenticated and successfully used in their previous study AAV production and purification were performed by ChuangRui Bio Lianyungang, China. Culture media were replaced with DMEM plus 0. Cell lysates were gently transferred onto the top layer, and the remaining volume of the ultracentrifuge tube was filled with cell lysis buffer. The viral titer was determined by qPCR assay with a standard curve generated by serial dilutions of the AAV shuttle vector. Mice were granted free access to pre-chilled food and water during the whole assay. Core body temperature was monitored at indicated time points using a portable intelligent digital thermometer TH During this period, metabolic parameters including mouse body temperature, body weight, blood glucose, as well as plasma TG and NEFA levels were monitored. Body surface temperature was measured using a thermal imaging camera FLIR Systems, Tsc InfraRed Camera. Mice were anesthetized with isoflurane and quietly laid on a whiteboard with their back up, followed by image capturing with the camera anchored at the same height for all mice in the same batch. FLIR Tools 5. Food and water were freely accessible to mice. O 2 consumption, CO 2 production, energy expenditure, total locomotor activity, and food intake were monitored under both normal conditions and under adrenergic stimulation. The metabolic parameters for each mouse were measured two days before CL injection and were continued to be monitored for one more day after CL injection. Mouse running performance was assessed using the treadmill running system from Columbus Instruments. The inclination angle was level. Total running distance and running time were recorded at the point when the mice reached exhaustion. After full differentiation, myotubes were subject to RNA isolation and gene expression analysis. ExpiF cells were transfected with pcDNA3. The XF96 microplate was then loaded into a Seahorse XFe96 analyzer for equilibration and determination of the basal respiration rate. OCRs were recorded and the ATP production, maximal respiration, and the spare respiratory capacity-dependent OCRs were calculated. Cell viability was also quantified using the CCK8 kit Beyotime for normalization. The XF Cell Mito Stress test kit Agilent was used in this assay. Seahorse Wave Desktop and Controller 2. An NMR analyzer NIUMAG, QMNH was applied to measure the body fat and lean mass. An enzyme-linked immunosorbent assay ELISA kit from Crystal Chem was used to measure plasma insulin concentration. For the detection of Musclin levels in human plasma, a Human Musclin ELISA kit CUSABIO, CSB-Eh was applied. For the detection of ANP levels in mouse plasma, a mouse ANP ELISA kit Elabscience, E-EL-Mc was used. For the detection of CNP levels in mouse plasma, a Mouse CNP ELISA kit Beijing Sino-UK Institute of Biological Technology, HY-NE was applied. Mouse tissues including liver, skeletal muscle, BAT, eWAT, and iWAT were dissected, fixed in formalin, followed by embedding in paraffin and cutting for tissue sections at μm for liver, skeletal muscle, and BAT, or μm for eWAT and iWAT. Tissue sections were then stained with hematoxylin and eosin, and subjected to image acquisition using NIS Elements F 4. The cell size of iWAT was quantified using Image J 1. The SEAP-binding assay was performed as described Firstly, the vectors expressing SEAP or SEAP-Musclin fusion protein were transiently transfected into HEKT cells. The sections were washed with PBS containing 0. CellSens Standard Olympus was used for image acquisition and data collection. Total RNA from WAT was isolated using a commercially available kit TIANGEN Biotech, DP Total RNA from other tissues including skeletal muscle, heart, brain, small intestine, liver, BAT, bone, and cultured cells was isolated following the standard method using TRIzol. RNA was then reverse transcribed using HiScript II Q RT SuperMix Vazyme, R , followed by qPCR analysis using SYBR Green Roche. The qPCR primers used are listed in Supplementary Data. Sequencing libraries were constructed from total RNA using SMART-RNAseq Library Prep Kit Hangzhou KaiTai, AT In brief, mRNA was isolated from total RNA with Sera-Mag Magnetic Olido dT particles, and then chemically fragmented. And the cDNA libraries were subsequently amplified using the KAPA high-fidelity DNA polymer. Quality of the libraries was validated by the Bioanalyzer Agilent Technologies. Subsequently, high-throughput sequencing was performed using a NovaSeq Illumina. Raw reads were filtered with fastp V0. Filtered data were then aligned with HISAT2 V2. p13 for human data. The FPKM fragments per kilobase of exon per million fragments mapped was calculated using StringTie V2. For iWAT from cold-acclimated and room temperature-housed control WT mice, RNA sequencing was performed in Majorbio Bio-pharm Biotechnology Co. Shanghai, China. The library was prepared using TruSeqTM RNA Sample Prep Kit Illumina. Shortly, mRNA was isolated by oligo dT beads and then fragmented. After quantified by TBS, paired-end RNA sequencing was performed using the NovaSeq Illumina. The raw reads were processed similarly and the TPM transcripts per million reads values were used to determine gene expression levels. For human scWAT, RNA sequencing was performed in BGI Shenzhen, China. Similarly, mRNA was purified using oligo dT -attached magnetic beads and was then fragmented. cDNA was generated by random hexamer-primed reverse transcription, and end-repaired through incubation with a-Tailing Mix and RNA Index Adapters. The obtained cDNA fragments were amplified using PCR followed by purification with Ampure XP Beads, which was validated on the Bioanalyzer Agilent Technologies. Distinctively, further heat-denaturation and circularization by the splint oligo sequence of the PCR products from previous step were needed to get the final library. Pair end base reads were generated for analysis. The sequencing data was filtered with SOAPnuke V1. p12 with HISAT2 V2. Expression level of gene FPKM value was calculated by RSEM V1. For all these sequencing data, differential expression analysis was performed using the Deseq2 v. Gene Ontology GO and pathway grouping and enrichment studies were performed by clusterProfiler V3. Html Results were visualized by ggplot2 V3. html 70 and pheatmap V1. Protein lysates from skeletal muscles and adipose tissues, or whole-cell lysates from cultured cells were quantified using BCA protein assay Beyotime. The final results were visualized with chemiluminescence ECL western blotting substrates. A proximity-dependent biotin identification BioID assay was used to identify the receptors for Musclin on the plasma membrane of adipocytes. The agarose beads were pelleted by centrifugation and then subjected to five times washing with washing buffer, followed by protein denaturation, SDS-PAGE gel electrophoresis, and Coomassie blue staining or silver staining Pierce Silver Stain Kit , Thermo Fisher. The gel fragments in pulldown samples containing differential protein bands as compared to control were collected for electrospray ionization tandem M. analysis on a Thermo Finnigan LTQ Orbitrap Instrument Proteome Discoverer version 1. The mass spectrometry proteomic data have been deposited to the ProteomeXchange Consortium via the PRIDE 74 partner repository with the dataset identifier PXD HEKT cells were transiently transfected with plasmids expressing control or Musclin. Finally, cells and culture media were harvested separately. Cells were subjected to total protein lysate preparation. The obtained protein pellet was washed in cold acetone, air-dried, and finally resuspended in SDS-containing lysis buffer. The input and IP samples were subjected to immunoblotting using antibodies against Musclin Abcam , Flag Sigma, A, M2 , or HSL Cell signaling, s. Cell proteins were quantified using a BCA protein assay kit Beyotime for normalization. Tissue weight was used for normalization. A standard chromogen method was used to measure the tissue non-heme iron described as previously described All statistical analyses were performed using GraphPad Prism 9 software. n values represent biological replicates for cell experiments, or mouse number for in vivo animal studies, or human subject number unless otherwise indicated. Specific details for the n value are noted in each figure legend. Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. RNA-Seq data of iWAT from cold-acclimated mice and their controls are available in SRA database under accession code: PRJNA The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE 74 partner repository with the dataset identifier PXD , which can be freely accessed. The images representing human participants, mouse models, skeletal muscle, myotube, adipose tissue, RNA-Sequencing, qPCR analysis, culture dish, and adipocyte shown in Figs. Source data are provided with this paper. Meng, Z. et al. Glucose sensing by skeletal myocytes couples nutrient signaling to systemic homeostasis. Cell 66 , — e Article CAS PubMed PubMed Central Google Scholar. Baf60c drives glycolytic metabolism in the muscle and improves systemic glucose homeostasis through Deptor-mediated Akt activation. Diabetes 63 , — Uncoupling exercise bioenergetics from systemic metabolic homeostasis by conditional inactivation of Baf60 in skeletal muscle. Diabetes 67 , 85—97 Article CAS PubMed Google Scholar. Pedersen, B. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Febbraio, M. Who would have thought—myokines two decades on. Article PubMed Google Scholar. Whitham, M. Extracellular vesicles provide a means for tissue crosstalk during exercise. Cell Metab. Giudice, J. Muscle as a paracrine and endocrine organ. Article CAS Google Scholar. Priest, C. Inter-organ cross-talk in metabolic syndrome. Ishibashi, J. Beige can be slimming. Science , — Petrovic, N. Chronic peroxisome proliferator-activated receptor gamma PPARgamma activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. Rosen, E. What we talk about when we talk about fat. Cell , 20—44 Ikeda, K. The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Wu, J. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell , — Matthew, H. Brown and beige fat: development, function and therapeutic potential. Article Google Scholar. Townsend, K. Brown fat fuel utilization and thermogenesis. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Med 23 , — Kaisanlahti, A. Browning of white fat: agents and implications for beige adipose tissue to type 2 diabetes. Biochem 75 , 1—10 Carobbio, S. Brown and beige fat: From molecules to physiology and pathophysiology. Acta Mol. Cell Biol. lipids , 37—50 Blüher, M. Obesity: global epidemiology and pathogenesis. Wang, Q. The hepatokine Tsukushi gates energy expenditure via brown fat sympathetic innervation. Article PubMed PubMed Central Google Scholar. Xiong, X. Landscape of intercellular crosstalk in healthy and NASH liver revealed by single-cell secretome gene analysis. Cell 75 , — Thomas, G. Osteocrin, a novel bone-specific secreted protein that modulates the osteoblast phenotype. Nishizawa, H. Musclin, a novel skeletal muscle-derived secretory factor. Kazak, L. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Roux, K. A promiscuous biotin ligase fusion protein identifies proximal and interacting proteins in mammalian cells. Li, J. CAS PubMed Google Scholar. Tsai, T. Sim, J. P2X1 and P2X4 receptor currents in mouse macrophages. Shen, W. Interaction of rat hormone-sensitive lipase with adipocytelipid-binding protein. Natl Acad. USA 96 , — Article ADS CAS PubMed PubMed Central Google Scholar. Bostrom, P. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature , — Article ADS PubMed PubMed Central Google Scholar. Rao, R. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Roberts, L. β-Aminoisobutyric acid induces browning of white fat and hepatic β -oxidation and is inversely correlated with cardiometabolic risk factors. Moffatt, P. Osteocrin is a specific ligand of the natriuretic peptide clearance receptor that modulates bone growth. Watanabe-Takano, H. Mechanical load regulates bone growth via periosteal Osteocrin. Cell Rep. Zhou, R. Endocrine role of bone in the regulation of energy metabolism. Bone Res. Ataman, B. Evolution of Osteocrin as an activity-regulated factor in the primate brain. Subbotina, E. Musclin is an activity-stimulated myokine that enhances physical endurance. USA , — Szaroszyk, M. Skeletal muscle derived Musclin protects the heart during pathological overload. Li, Y. Role of musclin in the pathogenesis of hypertension in rat. PLoS One 8 , e Miyazaki, T. A new secretory peptide of natriuretic peptide family, osteocrin, suppresses the progression of congestive heart failure after myocardial infarction. Hu, C. Journal of the International Society of Sports Nutrition , 5 doi Pre-workout consumption of Celsius® enhances the benefits of chronic exercise on body composition and cardiorespiratory fitness. Abbie E Smith, Jennifer L Graef, Kristina L Kendall, Travis W Beck and Joel T Cramer. Journal of the International Society of Sports Nutrition , 5 Suppl 1 :P8 doi Low-calorie thermogenic beverage and exercise improves composition and lipid profile in overweight and obese women. Abbie E. Smith, Jordan R. Moon, Chris M. Lockwood,, Kristina L. Kendall, David H. Low-Calorie energy drink improves physiological response to exercise in previously sedentary men: A placebo controlled efficacy and safety study. Christopher M. Lockwood, Jordan R. Moon, Abbie E. Smith, Sarah E. Tobkin, Kristina L. Kendall, Jennifer L. Graef, Joel T. Cramer, and Jeffrey R. Journal of Strength and Conditioning Research Aug;24 8 doi: Hi This is really helpful article I was searching for info about liposuction but when I came across this article and read it, it has changed my view. Now I know another safe way to reduce fat. thanks for this article. That is one impressive post, it highlights a lot of useful information for anyone building up muscle or trying to lose weight. Be the first to know about exclusive. content, deals and promotions. How Thermogenic Supplements Affect Fat Loss, Muscle Gain and Athletic Performance by Fitness Contributor Oct 1, Debbie J. Fat Loss Greater stored fat was used as an energy source for a single dose. Muscle Gain A few studies showed greater muscle mass gains with thermogenic use compared to controls. |
Wacker, Sie hat der einfach ausgezeichnete Gedanke besucht
Entschuldigen Sie, dass ich mich einmische, aber mir ist es etwas mehr die Informationen notwendig.
Ich meine, dass es das sehr interessante Thema ist. Ich biete Ihnen es an, hier oder in PM zu besprechen.
der sehr wertvolle Gedanke