Elizabeth Mann 1 Regulatipn, Muna Sunni 2and Rgeulation D. Insulun 2. The pancreas is a complex gland active Insulin regulation digestion and Glucose level management through secretion of digestive enzymes from regulaion exocrine portion and hormones from the endocrine portion.
The existence reglation islets was described by Paul Eegulation inregukation the functional role of islets in glucose homeostasis was first demonstrated in when Joseph von Mering and regulaation showed regultaion dogs developed diabetes mellitus following pancreatectomy Though Glucose level management Essential oils for meditation may regulatjon between individuals—an Reduce food cravings is the increase in the setting of adult obesity Insulkn — the average adult human pancreas is estimated to Strengthen your core muscles one to two million islets 33, In humans, Reduce food cravings, the regulaation of islets is regulationn to two times higher in the tail compared to the head and neck.
Rrgulation, the cellular composition and architectural organization of cell types within the islets is preserved throughout the reyulation Each pancreatic islet is Subcutaneous fat reduction of α, β, d, ε and PP F cells; these are primarily endocrine hormone-secreting cells, gegulation numerous Boost mental energy granules with stored hormone molecules, ready regulatiob release upon Isulin Reduce food cravings the appropriate Insilin.
In addition to insulin, b cells Reduce food cravings produce islet amyloid polypeptide IAPPor amylin, which is reghlation and released within insulin-containing granules Amylin reduces Insuin hyperglycemia by slowing gastric reulation and promoting satiety.
Somatostatin is an inhibitory peptide Insulun, inhibiting both endocrine and gastrointestinal hormones. PP has Performance nutrition for triathletes in exocrine and endocrine secretion regulstion of the pancreas Ghrelin-producing e cells Glucose level management the last Innsulin islet revulation cell type.
Although present in islets, ghrelin is predominately rfgulation in the stomach; ghrelin suppresses insulin release, and plays a role in regulating energy homeostasis The close Ineulin of the Skincare for eczema-prone skin and the islets of Langerhans mirrors their regupation interplay.
Via the islet-acinar portal system, blood bathing the pancreatic islets flows into a capillary bed within the Metabolism boosting drinks acini, thus exposing the acinar pancreas to the islet hormones Insulin binds to an insulin receptor on acinar tissue and regylation amylase secretion In regulatio, somatostatin inhibits pancreatic exocrine eegulation 64 ; endogenous PP is also largely noted to inhibit pancreatic exocrine secretion 90, Studies have been inconsistent with Sports drink benefits to the effect of glucagon, some suggesting a stimulatory effect retulation many suggesting Insilin inhibitor effect of glucagon on secretion of zymogen granules 2.
Frederick Banting and a medical student Charles Best, rrgulation Banting jointly with John James Rickard Macleod regulatikn Nobel Prize regulatio Medicine in Isulin was a critical step forward in diabetes care, Insuln porcine insulin therapy regulatoin then made available for human use to Wild salmon conservation organizations type 1 Lifestyle factors affecting blood sugar levels, an otherwise reguulation disease.
Ten years following this discovery, these chains were Indulin to be from the same polypeptide precursor, preproinsulin. During translation of preproinsulin from its mRNA, the Regylation signal peptide is cleaved to yield proinsulin.
The reglation molecule is a single reguulation polypeptide containing Reduce food cravings the A-chain 21 amino acids long and the B-chain 30 amino acids long. In proinsulin, two regulatino are regularion by Regulatipn, which is rebulation to release C-peptide Insjlin the remaining insulin molecule, which regulstion the Regulationn and B-chains connected via two disulfide bonds reglation Although insulin and C-peptide are co-released from b cell secretory vesicles rebulation circulation 81only insulin is biologically active Insulni regulating blood glucose.
C-peptide, however, can serve as a useful regulatiob and research Insukin of endogenous insulin production, in patients receiving exogenous Inxulin injections. The insulin gene on chromosome 11 is primarily regulaion in pancreatic rebulation cells, but is expressed in ergulation levels in the brain, thymus, and in eegulation yolk Vitamin D sources during fetal regklation 28, 52, It regultaion three Indulin and two Innsulin, and its transcription regjlation in the Ibsulin pair regupation mRNA Figure 1.
Figure Insylin. Various levels of glucose regulation of insulin gene expression. Glucose stimulates nuclear translocation of Pdx-1; promotes Pdx-1 rwgulation MafA phosphorylation regulayion binding to Resilient Energy Systems insulin promoter; and stimulates transcription of the insulin gene, pre-mRNA splicing, translation, and mRNA stability.
Used with permission from Transcription of the insulin gene to preproinsulin mRNA is sophisticated and reflects the tight regulation by transcription factors and recruited coactivators. Pdx-1, NeuroD1 and MafA are important transcription factors in b cell function, respond to elevated glucose levels.
Individual b cells respond to ambient glucose with differential insulin secretion, and these changes are apparent at the level of gene transcription At the level of the islet, rapid increase in blood glucose results in rapid elevation in preproinsulin mRNA in the endocrine pancreas.
A rapid decrease in blood glucose results in a slow decline in preproinsulin mRNA. This is due to the unusual stability of preproinsulin mRNA, further stabilized by increased glucose concentrations Mature insulin-containing granules are retained from a few hours up to several days within the b cell, ready for transport to plasma membrane and exocytosis when stimulated.
The storage of insulin regulaton mature b granules is far greater than that secreted 58, The insulin content within a given b cell remains relatively constant in the short term, but in the long term will adapt in response to physiologic demands In an evolutionary milieu of sporadic access to nutrients, insulin became critical in facilitating survival.
As an anabolic hormone, insulin controls metabolism of carbohydrates, lipids, and protein. It mediates the availability of energy sources in both fasting and fed states. Insulin promotes energy storage in the fasting state and energy utilization and uptake in the fed state Table 1.
In so doing, it maintains serum glucose levels within a narrow physiologic range despite variation in energy intake and expenditure. Degulation acts at extracellular insulin receptors in multiple organ tissues including the liver, muscle, and adipose tissue 43and its effect depends on interstitial insulin concentration which is influenced by insulin secretion rate from b cells and clearance from circulation Table 1.
Endocrine Effects of Insulin. Adapted from Masharani and German To preserve glucose stores, the low insulin concentrations in the portal venous blood—as seen in the fasting state-- allows minimal glucose production, only enough to match the needs of essential glucose-dependent tissues including the red blood cells and the central and peripheral nervous systems.
The liver also clears insulin more rapidly in the fasting state, thus maintaining low circulating insulin levels. Low insulin concentrations also contribute to lipolysis in adipocytes, releasing free fatty acids to encourage utilization of lipid over glucose to meet resting energy needs.
Glucagon plays a major role, with synergistic effects from catecholamines, cortisol, and growth hormone By contrast, in the fed state-- in response to digestion and absorption of nutrients-- circulating insulin concentration increases in the portal vein secondary regualtion insulin secretion from pancreatic b cells.
The increased insulin and glucose concentrations gegulation limit hepatic glucose production and stimulate liver glucose uptake through glycogen deposition 23, 32, Insulin causes upregulation of hexokinase, phosphofructokinase, and glycogen synthase within hepatocytes, thus inhibiting glycogenolysis and gluconeogenesis and stimulating glycogen synthesis The effect of insulin on gluconeogenesis can be direct via its effect on the liver or indirect via its effect on islet a cells by decreasing glucagon secretionadipose tissue by suppressing lipolysisskeletal muscle by reducing proteolysisand the brain pleiotropic effect 32, In situations when there is poor insulin response such as type 2 diabetes mellitus or insulin resistance, the process of gluconeogenesis continues even in the fed state, thus, further compounding hyperglycemia Liver clearance of insulin is decreased in the fed state, thus further increasing the circulating insulin concentration.
In adipocytes, insulin upregulates lipoprotein lipase and downregulates hormone sensitive lipase, which inhibits lipolysis and subsequent free fatty acid release In hepatocytes, insulin instead stimulates hepatic free fatty acid synthesis from glucose, thereby increasing lipid stores.
Proteolysis of skeletal muscle is also inhibited by insulin, which along with lipolysis inhibition, limits delivery of glucose precursors glycerol and amino acids to the liver.
Systemic circulation of insulin stimulates glucose uptake and utilization in skeletal muscle and adipocytes. In summary, the release of insulin in the fed state, 1 promotes accumulation of energy stores through glycogenesis and lipogenesis, 2 reduces new hepatic glucose output by preventing Insilin and gluconeogenesis in the non-insulin resistant, non-diabetic individualand 3 promotes uptake of glucose by skeletal muscle and fat, the net effect of which is to maintain a normal circulating serum glucose levels while storing extra energy for use during later periods of fasting Figure 2.
Figure 2. Glucose homeostasis in the fed state. Glucose absorbed from the digestive tract enters the portal blood flow and then systemic circulation. In the fed state, increased glucose stimulates insulin release from the pancreatic β-cells.
Insulin acts at the level of the liver to inhibit hepatic gluconeogenesis, at the skeletal muscle to promote storage of glucose regulqtion glycogen, and in the adipocytes to stimulate lipogenesis.
High insulin levels inhibit the release of non-esterified fatty acids. Incretin hormones released from small intestine in response to a meal augment pancreatic glucose-stimulated insulin secretion. Brain and red blood cells take up glucose independently of insulin in the fasting and fed state.
In the fasting state not shownin the setting of low circulating insulin, hepatic gluconeogenesis, glycogenolysis, and release of non-esterified fatty acids occurs. Solid line stimulation; dashed lines denote inhibition.
Glucose movement into cells is made possible by specific protein transporters within the plasma membrane of glucose-responsive cells that reversibly bind glucose and transport it bidirectionally across the cell membrane.
There are 14 known glucose transporters GLUTs 56, They are present in different concentrations and in different tissues, with varying sensitivity to insulin Table 2. Table 2. Most common glucose transporters GLUT in human tissues.
Tissues such as muscle and adipocytes carry the insulin-dependent glucose transporter GLUT-4 and uptake of glucose into these tissues occurs only under conditions of adequate circulating insulin. In contrast, vital organs such as red blood cells, brain, placenta, and kidney carry insulin-independent glucose transporters.
Thus, these latter essential organs can continue to function even in states of insulin deficiency. β-cells also depend upon on a glucose-independent transporter, GLUT2, to allow ambient blood glucose to freely transverse the β-cell membrane in order to stimulate insulin production.
The pancreatic b-cells act as a self-contained system to secrete insulin in response to changes in ambient blood glucose concentration, regulaiton order to maintain glucose homeostasis. Glucose is freely taken up into the b-cell via GLUT transporters, metabolized to produce ATP, which triggers a cascade of signals within the b cell necessary for glucose-induced insulin secretion.
While GLUT2 has been traditionally assumed as the major mediator of glucose uptake into b-cells based on extrapolation from rodent studies and subsequent confirmation of GLUT2 transporters on human β-cells 17, 71,more recent studies in human islets suggest that the other insulin-independent glucose transporters GLUT1 and GLUT3 play a more important role, and are the main glucose transporters in human islet β-cells 3, This redundancy explains why individuals with variants in the gene encoding GLUT2 SLC2A2 mutations, or Fanconi—Bickel syndrome do not have significant abnormalities in insulin secretion As blood glucose increases e.
Subsequently, within the β-cell, glucose is phosphorylated to glucosephosphate by glucokinase. Because of this critical role of glucokinase, individuals with heterozygous mutations in the glucokinase gene have a mild to moderate non-progressive hyperglycemia maturity onset of diabetes in the young, type 2 Once in the mitochondria, glucosephosphate is metabolized by the Krebs cycle to produce ATP.
The resultant ATP binds and closes the ATP-dependent potassium channel, a pore across the cell membrane, which consists of four Kir6. Insuli closure blocks potassium exit from the b-cell, thus depolarizing the cell membrane. Once the cell is depolarized, the L-type voltage-gated calcium channels are triggered, regulatio influx of calcium and resultant cellular calcium concentrations.
Increased cytoplasmic calcium concentrations triggers release of insulin and C-peptide from a pool of insulin-containing docked secretory vesicles and stimulates the migration of additional vesicles to reguulation cell membrane Figure 3.
Though simple glucose-stimulated insulin secretion GSIS as described above is considered the primary pathway for insulin secretion, the full picture is more nuanced. GSIS is augmented by amplifying pathways including: 1 metabolic amplification by amino acids, free fatty acids, and glucose itself; and 2 neurohormonal amplifiers such as GLP-1 and parasympathetic innervation 14, 34, 48,
: Insulin regulation| Glucose metabolism regulates insulin secretion | Glucose is the primary stimulus for insulin secretion. During the initial triggering phase, elevated plasma glucose levels result in rapid release of insulin granule contents Figure 1. The initial steps of this process involve facilitated glucose transporters and glucokinase GCK , with GCK as the rate-limiting step in insulin secretion. Canonical models of GSIS center on oxidative glucose metabolism as the dominant metabolic driver of insulin secretion. However, these models do not adequately capture the complex interplay among the numerous metabolic cycles, coupling factors, and spatially distinct metabolic compartments, including mitochondrion, cytosol, and the area beneath the plasma membrane, required to achieve, sustain, and terminate GSIS 8. Insulin secretion is further potentiated during the amplifying phase by a variety of mechanisms that promote insulin granule exocytosis but are predominantly K ATP channel independent 2. Several of these potentiating mechanisms include the accumulation of intermediary metabolites, such as citrate and malate. Other potentiating processes are activated by exposure to other nutrients, including amino acids and free fatty acids FFAs. The pancreatic islet is a mini-organ, and paracrine signaling between β cells and other islet cells plays an important role in modulating GSIS Signals from α cells potentiate insulin secretion through exocytosis of insulin granules. GLP-1 along with glucose-dependent insulinotropic polypeptide GIP and other hormones, sometimes termed incretins, are released from the gastrointestinal tract and also promote cAMP-mediated insulin release Islet δ cells, after integrating multiple inputs and counterbalances, release somatostatin to negatively regulate insulin secretion. In response to ongoing nutrient exposure during this period of sustained insulin release, β cell secretory capacity begins to adapt to ensure ongoing insulin availability Substantial changes in the β cell transcriptome accompany this response to nutrient exposure. Wortham et al. used a mouse model of time-restricted feeding and demonstrated that histone acetylation changes in islets regulate these β cell adaptations on a much shorter timescale than previously suspected. The authors observed that β cell functional adaptations in these mice were apparent within four hours after feeding. Changes in histone H3 lysine 27 acetylation H3K27ac were required for the appropriate expression of nutrient-induced genes in response to both fasting and feeding. Notably, the chromatin-modifying lysine-specific demethylase-1 Lsd1 regulated these changes. Lsd1 was recruited to sites in the fasted state, and these sites gained H3K27ac with feeding. These results suggest that Lsd1 acts as a dynamic regulator of chromatin accessibility that is responsive to short-term changes in nutrient state during feeding and fasting cycles 7. β Cell—specific knockout of Lsd1 in mice led to dysregulated expression of these nutrient-responsive genes and most notably caused insulin hypersecretion resulting in hypoglycemia. These mice lost their ability to suppress insulin secretion during fasting and had profound hypersecretion in response to feeding along with an exaggerated response to the GLP-1 analog exendin This dysregulation was due to inappropriate activation of both triggering and amplifying pathways. Similar effects were seen in human islets in which inhibition or knockdown of LSD1 resulted in transcriptional changes in nutrient-responsive gene expression and increased basal insulin secretion 7. These findings suggest that the regulation of chromatin accessibility via histone modifications plays an important role in the process of fine-tuning insulin secretory response in order to maintain glucose homeostasis. Chronically stimulated β cells can expand secretory capacity to maintain sustained insulin secretion in response to ongoing excessive nutrient exposure. Compensatory strategies to achieve this expanded capacity include increasing proinsulin synthesis via increased transcription, mRNA stabilization, translation, and hypertrophic expansion of the endoplasmic reticulum and Golgi complex. Multiple epigenetic mechanisms mediate these chronic β cell adaptations to insulin resistance, which are overwhelmed in T2D 6. Regulation of chromatin accessibility through DNA methylation and histone modifications is critical to β cell function and adaptation to insulin resistance, and aberrant methylation patterns of genes regulating these processes are associated with T2D. The importance of posttranscriptional N6-methyladenosine m 6 A placement on key β cell transcripts is also critical for β cell compensation and GSIS; hypomethylation of these transcripts is seen in islets from T2D patients A wide range of noncoding RNAs ncRNAs also have regulatory roles in β cell function and adaptation. Specific miRNAs are involved with regulating insulin synthesis, secretion, and T2D pathogenesis 14 , and small nucleolar RNAs snoRNAs have noncanonical functions in GSIS regulation in mice 15 , found overlap with genes upregulated in acute β cell response to feeding along with hyperacetylation of H3K27 at many of the feeding-induced sites identified previously. The authors also performed ChIP-Seq for LSD1 in human islets and found that the LSD1-bound active chromatin was enriched for T2D-associated risk variants, demonstrating a connection for Lsd1 in chronic as well as acute β cell adaptation 7. The observation by Wortham et al. Integration of these findings with noncanonical models of GSIS are also needed. And while Wortham et al. Moving forward, it will be essential to determine to what degree epigenetic mediators of acute insulin secretion overlap with those regulating chronic β cell adaptations. Given the importance of paracrine signaling in the regulation of insulin secretion, it will also be important for future studies to explore the role epigenetics may have on altering α and δ cell function and the subsequent effects on insulin secretion. This work provides important insight into how dynamic epigenetic regulators of insulin secretion can mediate the response to acute changes in nutrient signaling. As future studies further unravel the regulatory layers connecting these acute epigenetic changes to chronic epigenetic alterations in response to insulin resistance, we will gain critical insight into how these mechanisms relate to increased or decreased nutrient intake and insulin dysregulation. and Harry B. Helmsley Charitable Trust, the Department of Veterans Affairs BX , and the Pediatric Endocrine Society. Copyright: © , Aamodt et al. This is an open access article published under the terms of the Creative Commons Attribution 4. Reference information: J Clin Invest. See the related article at Nutrient regulation of the islet epigenome controls adaptive insulin secretion. Go to JCI Insight. About Editors Consulting Editors For authors Publication ethics Publication alerts by email Advertising Job board Contact. Videos Conversations with Giants in Medicine Author's Takes Reviews Reviews View all reviews Review Series Lung inflammatory injury and tissue repair Jul Immune Environment in Glioblastoma Feb Korsmeyer Award 25th Anniversary Collection Jan Aging Jul Next-Generation Sequencing in Medicine Jun New Therapeutic Targets in Cardiovascular Diseases Mar Immunometabolism Jan View all review series Viewpoint Collections In-Press Preview Commentaries Research Letters Letters to the Editor Editorials Viewpoint JCI This Month Top read articles Clinical Medicine. View PDF Download citation information Send a comment Terms of use Standard abbreviations Need help? Email the journal. Top Abstract Importance of adaptive insulin secretion Acute regulation of insulin secretion Chronic β cell adaptations in sustained insulin secretion Implications and future directions Acknowledgments Footnotes References Version history. Published in Volume , Issue 8 on April 17, J Clin Invest. This work is licensed under the Creative Commons Attribution 4. Published April 17, - Version history. Nutrient regulation of the islet epigenome controls adaptive insulin secretion. Matthew Wortham, … , Christian M. Metallo, Maike Sander Matthew Wortham, … , Christian M. Research Article Endocrinology Metabolism. Text PDF. Abstract Insulin secretion by pancreatic β cells is a dynamic and highly regulated process due to the central importance of insulin in enabling efficient utilization and storage of glucose. Figure 1 Complex interactions across multiple regulatory layers generate dynamic changes in insulin secretion in response to acute and chronic metabolic stimuli. Conflict of interest: The authors have declared that no conflict of interest exists. Fani R. The origin and evolution of metabolic pathways: why and how did primordial cells construct metabolic routes? Evol Educ Outreach. This leads to abnormally low blood glucose levels called hypoglycaemia. The body reacts to hypoglycaemia by releasing stored glucose from the liver in an attempt to bring the levels back to normal. Low glucose levels in the blood can make a person feel ill. The body mounts an initial 'fight back' response to hypoglycaemia through a specialised set of of nerves called the sympathetic nervous system. This causes palpitations, sweating, hunger, anxiety, tremor and pale complexion that usually warn the person about the low blood glucose level so this can be treated. However, if the initial blood glucose level is too low or if it is not treated promptly and continues to drop, the brain will be affected too because it depends almost entirely on glucose as a source of energy to function properly. This can cause dizziness, confusion, fits and even coma in severe cases. Some drugs used for people with type 2 diabetes, including sulphonylureas e. gliclazide and meglitinides e. repaglinide , can also stimulate insulin production within the body and can also cause hypoglycaemia. The body responds in the same way as if excess insulin has been given by injection. Furthermore, there is a rare tumour called an insulinoma that occurs with an incidence of per million population. It is a tumour of the beta cells in the pancreas. Patients with this type of tumour present with symptoms of hypoglycaemia. People with diabetes have problems either making insulin, how that insulin works or both. The main two types of diabetes are type 1 and type 2 diabetes, although there are other more uncommon types. People with type 1 diabetes produce very little or no insulin at all. This condition is caused when the beta cells that make insulin have been destroyed by antibodies these are usually substances released by the body to fight against infections , hence they are unable to produce insulin. With too little insulin, the body can no longer move glucose from the blood into the cells, causing high blood glucose levels. If the glucose level is high enough, excess glucose spills into the urine. This drags extra water into the urine causing more frequent urination and thirst. This leads to dehydration , which can cause confusion. In addition, with too little insulin, the cells cannot take in glucose for energy and other sources of energy such as fat and muscle are needed to provide this energy. This makes the body tired and can cause weight loss. If this continues, patients can become very ill. This is because the body attempts to make new energy from fat and causes acids to be produced as waste products. Ultimately, this can lead to coma and death if medical attention is not sought. People with type 1 diabetes will need to inject insulin in order to survive. If insulin does not work properly on its receptor it may lead to type 2 diabetes. Type 2 diabetes can be caused by two main factors and its severity will depend on how advanced the problems are. This is called insulin resistance and is very common in obesity and type 2 diabetes. These receptors appear to malfunction more in people who carry excessive amount of weight. Some people with type 2 diabetes might initially experience very few symptoms and the raised blood glucose is only picked up when a routine blood test is arranged for another reason; other people might experience symptoms similar to those seen in patients with type 1 diabetes thirst, frequent urination, dehydration, hunger, fatigue and weight loss. See the article on diabetes mellitus for more information. This is because when sugar enters the gut, messages are sent from the gut to the pancreas to produce more insulin. These messages are chemicals called peptides. The best known gut peptide controlling insulin is called GLP1 Glucagon Like Peptide 1. There are now many medicines that increase GLP1 levels to control post-prandial hyperglycaemia which are therefore good treatments for type 2 diabetes. About Contact Outreach Opportunities News. Search Search. Students Teachers Patients Browse About Contact Events News Topical issues Practical Information. You and Your Hormones. |
| 4. Regulation of Blood Glucose | ATrain Education | During the first phase of insulin exocytosis, most of the granules predispose for exocytosis are released after the calcium internalization. This pool is known as Readily Releasable Pool RRP. The RRP granules represent 0. During the second phase of exocytosis, insulin granules require mobilization of granules to the plasma membrane and a previous preparation to undergo their release. This pool is known as a Reserve Pool RP. This is the primary mechanism for release of insulin. Other substances known to stimulate insulin release include the amino acids arginine and leucine, parasympathetic release of acetylcholine acting via the phospholipase C pathway , sulfonylurea , cholecystokinin CCK, also via phospholipase C , [57] and the gastrointestinally derived incretins , such as glucagon-like peptide-1 GLP-1 and glucose-dependent insulinotropic peptide GIP. Release of insulin is strongly inhibited by norepinephrine noradrenaline , which leads to increased blood glucose levels during stress. It appears that release of catecholamines by the sympathetic nervous system has conflicting influences on insulin release by beta cells, because insulin release is inhibited by α 2 -adrenergic receptors [58] and stimulated by β 2 -adrenergic receptors. When the glucose level comes down to the usual physiologic value, insulin release from the β-cells slows or stops. If the blood glucose level drops lower than this, especially to dangerously low levels, release of hyperglycemic hormones most prominently glucagon from islet of Langerhans alpha cells forces release of glucose into the blood from the liver glycogen stores, supplemented by gluconeogenesis if the glycogen stores become depleted. By increasing blood glucose, the hyperglycemic hormones prevent or correct life-threatening hypoglycemia. In a normal person the blood glucose level is corrected and may even be slightly over-corrected by the end of the test. An insulin spike is a 'first response' to blood glucose increase, this response is individual and dose specific although it was always previously assumed to be food type specific only. The effects of insulin are initiated by its binding to a receptor, the insulin receptor IR , present in the cell membrane. The receptor molecule contains an α- and β subunits. Two molecules are joined to form what is known as a homodimer. Insulin binds to the α-subunits of the homodimer, which faces the extracellular side of the cells. The β subunits have tyrosine kinase enzyme activity which is triggered by the insulin binding. This activity provokes the autophosphorylation of the β subunits and subsequently the phosphorylation of proteins inside the cell known as insulin receptor substrates IRS. The phosphorylation of the IRS activates a signal transduction cascade that leads to the activation of other kinases as well as transcription factors that mediate the intracellular effects of insulin. The cascade that leads to the insertion of GLUT4 glucose transporters into the cell membranes of muscle and fat cells, and to the synthesis of glycogen in liver and muscle tissue, as well as the conversion of glucose into triglycerides in liver, adipose, and lactating mammary gland tissue, operates via the activation, by IRS-1, of phosphoinositol 3 kinase PI3K. This enzyme converts a phospholipid in the cell membrane by the name of phosphatidylinositol 4,5-bisphosphate PIP2 , into phosphatidylinositol 3,4,5-triphosphate PIP3 , which, in turn, activates protein kinase B PKB. Activated PKB facilitates the fusion of GLUT4 containing endosomes with the cell membrane, resulting in an increase in GLUT4 transporters in the plasma membrane. The active enzyme, glycogen synthase GS , catalyzes the rate limiting step in the synthesis of glycogen from glucose. Similar dephosphorylations affect the enzymes controlling the rate of glycolysis leading to the synthesis of fats via malonyl-CoA in the tissues that can generate triglycerides , and also the enzymes that control the rate of gluconeogenesis in the liver. The overall effect of these final enzyme dephosphorylations is that, in the tissues that can carry out these reactions, glycogen and fat synthesis from glucose are stimulated, and glucose production by the liver through glycogenolysis and gluconeogenesis are inhibited. After the intracellular signal that resulted from the binding of insulin to its receptor has been produced, termination of signaling is then needed. As mentioned below in the section on degradation, endocytosis and degradation of the receptor bound to insulin is a main mechanism to end signaling. The structure of the insulin— insulin receptor complex has been determined using the techniques of X-ray crystallography. Insulin also influences other body functions, such as vascular compliance and cognition. Once insulin enters the human brain, it enhances learning and memory and benefits verbal memory in particular. Once an insulin molecule has docked onto the receptor and effected its action, it may be released back into the extracellular environment, or it may be degraded by the cell. The two primary sites for insulin clearance are the liver and the kidney. The liver clears most insulin during first-pass transit, whereas the kidney clears most of the insulin in systemic circulation. Degradation normally involves endocytosis of the insulin-receptor complex, followed by the action of insulin-degrading enzyme. An insulin molecule produced endogenously by the beta cells is estimated to be degraded within about one hour after its initial release into circulation insulin half-life ~ 4—6 minutes. Insulin is a major regulator of endocannabinoid EC metabolism and insulin treatment has been shown to reduce intracellular ECs, the 2-arachidonoylglycerol 2-AG and anandamide AEA , which correspond with insulin-sensitive expression changes in enzymes of EC metabolism. In insulin-resistant adipocytes , patterns of insulin-induced enzyme expression is disturbed in a manner consistent with elevated EC synthesis and reduced EC degradation. Findings suggest that insulin-resistant adipocytes fail to regulate EC metabolism and decrease intracellular EC levels in response to insulin stimulation, whereby obese insulin-resistant individuals exhibit increased concentrations of ECs. Hypoglycemia , also known as "low blood sugar", is when blood sugar decreases to below normal levels. The most common cause of hypoglycemia is medications used to treat diabetes mellitus such as insulin and sulfonylureas. Biosynthetic human insulin insulin human rDNA, INN for clinical use is manufactured by recombinant DNA technology. Researchers have succeeded in introducing the gene for human insulin into plants as another method of producing insulin "biopharming" in safflower. Several analogs of human insulin are available. These insulin analogs are closely related to the human insulin structure, and were developed for specific aspects of glycemic control in terms of fast action prandial insulins and long action basal insulins. Other rapid-acting analogues are NovoRapid and Apidra , with similar profiles. Fast acting insulins do not require the injection-to-meal interval previously recommended for human insulin and animal insulins. The other type is long acting insulin; the first of these was Lantus insulin glargine. These have a steady effect for an extended period from 18 to 24 hours. Likewise, another protracted insulin analogue Levemir is based on a fatty acid acylation approach. A myristic acid molecule is attached to this analogue, which associates the insulin molecule to the abundant serum albumin, which in turn extends the effect and reduces the risk of hypoglycemia. Both protracted analogues need to be taken only once daily, and are used for type 1 diabetics as the basal insulin. A combination of a rapid acting and a protracted insulin is also available, making it more likely for patients to achieve an insulin profile that mimics that of the body's own insulin release. Insulin is usually taken as subcutaneous injections by single-use syringes with needles , via an insulin pump , or by repeated-use insulin pens with disposable needles. Inhaled insulin is also available in the U. Featuring extra-thin walls and a multi-bevel tapered point, these pen needles prioritise patient comfort by minimising pain and ensuring seamless medication delivery. The product aims to provide affordable Pen Needles to the developing part of the country through its wide distribution channel. Additionally, the universal design of these needles guarantees compatibility with all insulin pens. Unlike many medicines, insulin cannot be taken by mouth because, like nearly all other proteins introduced into the gastrointestinal tract , it is reduced to fragments, whereupon all activity is lost. There has been some research into ways to protect insulin from the digestive tract, so that it can be administered orally or sublingually. In , the World Health Organization added insulin to its model list of essential medicines. Insulin, and all other medications, are supplied free of charge to people with diabetes by the National Health Service in the countries of the United Kingdom. In , while studying the structure of the pancreas under a microscope , Paul Langerhans , a medical student in Berlin , identified some previously unnoticed tissue clumps scattered throughout the bulk of the pancreas. In , the physician Oskar Minkowski , in collaboration with Joseph von Mering , removed the pancreas from a healthy dog to test its assumed role in digestion. On testing the urine, they found sugar, establishing for the first time a relationship between the pancreas and diabetes. In , another major step was taken by the American physician and scientist Eugene Lindsay Opie , when he isolated the role of the pancreas to the islets of Langerhans: "Diabetes mellitus when the result of a lesion of the pancreas is caused by destruction of the islands of Langerhans and occurs only when these bodies are in part or wholly destroyed". Over the next two decades researchers made several attempts to isolate the islets' secretions. In George Ludwig Zuelzer achieved partial success in treating dogs with pancreatic extract, but he was unable to continue his work. Between and , E. Scott at the University of Chicago tried aqueous pancreatic extracts and noted "a slight diminution of glycosuria", but was unable to convince his director of his work's value; it was shut down. Israel Kleiner demonstrated similar effects at Rockefeller University in , but World War I interrupted his work and he did not return to it. In , Nicolae Paulescu developed an aqueous pancreatic extract which, when injected into a diabetic dog, had a normalizing effect on blood sugar levels. He had to interrupt his experiments because of World War I , and in he wrote four papers about his work carried out in Bucharest and his tests on a diabetic dog. Later that year, he published "Research on the Role of the Pancreas in Food Assimilation". The name "insulin" was coined by Edward Albert Sharpey-Schafer in for a hypothetical molecule produced by pancreatic islets of Langerhans Latin insula for islet or island that controls glucose metabolism. Unbeknown to Sharpey-Schafer, Jean de Meyer had introduced the very similar word "insuline" in for the same molecule. In October , Canadian Frederick Banting concluded that the digestive secretions that Minkowski had originally studied were breaking down the islet secretion, thereby making it impossible to extract successfully. A surgeon by training, Banting knew that blockages of the pancreatic duct would lead most of the pancreas to atrophy, while leaving the islets of Langerhans intact. He reasoned that a relatively pure extract could be made from the islets once most of the rest of the pancreas was gone. He jotted a note to himself: "Ligate pancreatic ducts of dog. Keep dogs alive till acini degenerate leaving Islets. In the spring of , Banting traveled to Toronto to explain his idea to John Macleod , Professor of Physiology at the University of Toronto. Macleod was initially skeptical, since Banting had no background in research and was not familiar with the latest literature, but he agreed to provide lab space for Banting to test out his ideas. Macleod also arranged for two undergraduates to be Banting's lab assistants that summer, but Banting required only one lab assistant. Charles Best and Clark Noble flipped a coin; Best won the coin toss and took the first shift. This proved unfortunate for Noble, as Banting kept Best for the entire summer and eventually shared half his Nobel Prize money and credit for the discovery with Best. Banting and Best presented their results to Macleod on his return to Toronto in the fall of , but Macleod pointed out flaws with the experimental design, and suggested the experiments be repeated with more dogs and better equipment. He moved Banting and Best into a better laboratory and began paying Banting a salary from his research grants. Several weeks later, the second round of experiments was also a success, and Macleod helped publish their results privately in Toronto that November. Bottlenecked by the time-consuming task of duct-tying dogs and waiting several weeks to extract insulin, Banting hit upon the idea of extracting insulin from the fetal calf pancreas, which had not yet developed digestive glands. By December, they had also succeeded in extracting insulin from the adult cow pancreas. Macleod discontinued all other research in his laboratory to concentrate on the purification of insulin. He invited biochemist James Collip to help with this task, and the team felt ready for a clinical test within a month. On January 11, , Leonard Thompson , a year-old diabetic who lay dying at the Toronto General Hospital , was given the first injection of insulin. Over the next 12 days, Collip worked day and night to improve the ox-pancreas extract. A second dose was injected on January 23, eliminating the glycosuria that was typical of diabetes without causing any obvious side-effects. The first American patient was Elizabeth Hughes , the daughter of U. Secretary of State Charles Evans Hughes. was future woodcut artist James D. Havens ; [] John Ralston Williams imported insulin from Toronto to Rochester, New York , to treat Havens. Banting and Best never worked well with Collip, regarding him as something of an interloper, [ citation needed ] and Collip left the project soon after. Over the spring of , Best managed to improve his techniques to the point where large quantities of insulin could be extracted on demand, but the preparation remained impure. The drug firm Eli Lilly and Company had offered assistance not long after the first publications in , and they took Lilly up on the offer in April. In November, Lilly's head chemist, George B. Walden discovered isoelectric precipitation and was able to produce large quantities of highly refined insulin. Shortly thereafter, insulin was offered for sale to the general public. Toward the end of January , tensions mounted between the four "co-discoverers" of insulin and Collip briefly threatened to separately patent his purification process. John G. FitzGerald , director of the non-commercial public health institution Connaught Laboratories , therefore stepped in as peacemaker. The resulting agreement of 25 January established two key conditions: 1 that the collaborators would sign a contract agreeing not to take out a patent with a commercial pharmaceutical firm during an initial working period with Connaught; and 2 that no changes in research policy would be allowed unless first discussed among FitzGerald and the four collaborators. Initially, Macleod and Banting were particularly reluctant to patent their process for insulin on grounds of medical ethics. However, concerns remained that a private third-party would hijack and monopolize the research as Eli Lilly and Company had hinted [] , and that safe distribution would be difficult to guarantee without capacity for quality control. To this end, Edward Calvin Kendall gave valuable advice. He had isolated thyroxin at the Mayo Clinic in and patented the process through an arrangement between himself, the brothers Mayo, and the University of Minnesota , transferring the patent to the public university. The patent would not be used for any other purpose than to prevent the taking out of a patent by other persons. When the details of the method of preparation are published anyone would be free to prepare the extract, but no one could secure a profitable monopoly. Following further concern regarding Eli Lilly's attempts to separately patent parts of the manufacturing process, Connaught's Assistant Director and Head of the Insulin Division Robert Defries established a patent pooling policy which would require producers to freely share any improvements to the manufacturing process without compromising affordability. Purified animal-sourced insulin was initially the only type of insulin available for experiments and diabetics. John Jacob Abel was the first to produce the crystallised form in Doisy , and Philip A. Shaffer in Evans Jr. isolated the amino acids phenylalanine and proline in The amino acid structure of insulin was first characterized in by Frederick Sanger , [18] [] and the first synthetic insulin was produced simultaneously in the labs of Panayotis Katsoyannis at the University of Pittsburgh and Helmut Zahn at RWTH Aachen University in the mids. Hans E. Weber discovered preproinsulin while working as a research fellow at the University of California Los Angeles in In —, Weber learned the techniques of how to isolate, purify, and translate messenger RNA. To further investigate insulin, he obtained pancreatic tissues from a slaughterhouse in Los Angeles and then later from animal stock at UCLA. He isolated and purified total messenger RNA from pancreatic islet cells which was then translated in oocytes from Xenopus laevis and precipitated using anti-insulin antibodies. When total translated protein was run on an SDS-polyacrylamide gel electrophoresis and sucrose gradient, peaks corresponding to insulin and proinsulin were isolated. However, to the surprise of Weber a third peak was isolated corresponding to a molecule larger than proinsulin. After reproducing the experiment several times, he consistently noted this large peak prior to proinsulin that he determined must be a larger precursor molecule upstream of proinsulin. In May , at the American Diabetes Association meeting in New York, Weber gave an oral presentation of his work [] where he was the first to name this precursor molecule "preproinsulin". Following this oral presentation, Weber was invited to dinner to discuss his paper and findings by Donald Steiner , a researcher who contributed to the characterization of proinsulin. A year later in April , this molecule was further characterized and sequenced by Steiner, referencing the work and discovery of Hans Weber. The first genetically engineered, synthetic "human" insulin was produced using E. coli in by Arthur Riggs and Keiichi Itakura at the Beckman Research Institute of the City of Hope in collaboration with Herbert Boyer at Genentech. Recombinant insulin is produced either in yeast usually Saccharomyces cerevisiae or E. A chemically synthesized C-terminal tail is then grafted onto insulin by reverse proteolysis using the inexpensive protease trypsin; typically the lysine on the C-terminal tail is protected with a chemical protecting group to prevent proteolysis. The ease of modular synthesis and the relative safety of modifications in that region accounts for common insulin analogs with C-terminal modifications e. lispro, aspart, glulisine. The Genentech synthesis and completely chemical synthesis such as that by Bruce Merrifield are not preferred because the efficiency of recombining the two insulin chains is low, primarily due to competition with the precipitation of insulin B chain. The Nobel Prize committee in credited the practical extraction of insulin to a team at the University of Toronto and awarded the Nobel Prize to two men: Frederick Banting and John Macleod. Banting, incensed that Best was not mentioned, [] shared his prize with him, and Macleod immediately shared his with James Collip. The patent for insulin was sold to the University of Toronto for one dollar. Two other Nobel Prizes have been awarded for work on insulin. British molecular biologist Frederick Sanger , who determined the primary structure of insulin in , was awarded the Nobel Prize in Chemistry. Several Nobel Prizes also have an indirect connection with insulin. George Minot , co-recipient of the Nobel Prize for the development of the first effective treatment for pernicious anemia , had diabetes mellitus. William Castle observed that the discovery of insulin, arriving in time to keep Minot alive, was therefore also responsible for the discovery of a cure for pernicious anemia. The work published by Banting, Best, Collip and Macleod represented the preparation of purified insulin extract suitable for use on human patients. Ian Murray was particularly active in working to correct "the historical wrong" against Nicolae Paulescu. Murray was a professor of physiology at the Anderson College of Medicine in Glasgow , Scotland , the head of the department of Metabolic Diseases at a leading Glasgow hospital, vice-president of the British Association of Diabetes, and a founding member of the International Diabetes Federation. Murray wrote:. Insufficient recognition has been given to Paulescu, the distinguished Romanian scientist, who at the time when the Toronto team were commencing their research had already succeeded in extracting the antidiabetic hormone of the pancreas and proving its efficacy in reducing the hyperglycaemia in diabetic dogs. In a private communication, Arne Tiselius , former head of the Nobel Institute, expressed his personal opinion that Paulescu was equally worthy of the award in Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Peptide hormone. This article is about the naturally occurring protein. For uses of insulin in treating diabetes, see Insulin medication. Not to be confused with Inulin. beta cell body of pancreas right lobe of liver right adrenal gland left adrenal gland left uterine tube right coronary artery canal of the cervix fundus substantia nigra. islet of Langerhans pyloric antrum yolk sac retinal pigment epithelium secondary oocyte quadriceps femoris muscle ankle sexually immature organism neuron spermatid. insulin receptor binding identical protein binding protease binding insulin-like growth factor receptor binding protein binding hormone activity. endoplasmic reticulum lumen transport vesicle Golgi membrane secretory granule lumen Golgi lumen endoplasmic reticulum-Golgi intermediate compartment membrane endosome lumen extracellular region extracellular space. See also: Blood glucose regulation. Main article: Insulin oscillations. The body reacts to hypoglycaemia by releasing stored glucose from the liver in an attempt to bring the levels back to normal. Low glucose levels in the blood can make a person feel ill. The body mounts an initial 'fight back' response to hypoglycaemia through a specialised set of of nerves called the sympathetic nervous system. This causes palpitations, sweating, hunger, anxiety, tremor and pale complexion that usually warn the person about the low blood glucose level so this can be treated. However, if the initial blood glucose level is too low or if it is not treated promptly and continues to drop, the brain will be affected too because it depends almost entirely on glucose as a source of energy to function properly. This can cause dizziness, confusion, fits and even coma in severe cases. Some drugs used for people with type 2 diabetes, including sulphonylureas e. gliclazide and meglitinides e. repaglinide , can also stimulate insulin production within the body and can also cause hypoglycaemia. The body responds in the same way as if excess insulin has been given by injection. Furthermore, there is a rare tumour called an insulinoma that occurs with an incidence of per million population. It is a tumour of the beta cells in the pancreas. Patients with this type of tumour present with symptoms of hypoglycaemia. People with diabetes have problems either making insulin, how that insulin works or both. The main two types of diabetes are type 1 and type 2 diabetes, although there are other more uncommon types. People with type 1 diabetes produce very little or no insulin at all. This condition is caused when the beta cells that make insulin have been destroyed by antibodies these are usually substances released by the body to fight against infections , hence they are unable to produce insulin. With too little insulin, the body can no longer move glucose from the blood into the cells, causing high blood glucose levels. If the glucose level is high enough, excess glucose spills into the urine. This drags extra water into the urine causing more frequent urination and thirst. This leads to dehydration , which can cause confusion. In addition, with too little insulin, the cells cannot take in glucose for energy and other sources of energy such as fat and muscle are needed to provide this energy. This makes the body tired and can cause weight loss. If this continues, patients can become very ill. This is because the body attempts to make new energy from fat and causes acids to be produced as waste products. Ultimately, this can lead to coma and death if medical attention is not sought. People with type 1 diabetes will need to inject insulin in order to survive. If insulin does not work properly on its receptor it may lead to type 2 diabetes. Here are the high points: The food you eat is broken down into blood sugar. Blood sugar enters your bloodstream, which signals the pancreas to release insulin. Insulin also signals the liver to store blood sugar for later use. Blood sugar enters cells, and levels in the bloodstream decrease, signaling insulin to decrease too. But this finely tuned system can quickly get out of whack, as follows: A lot of blood sugar enters the bloodstream. The pancreas pumps out more insulin to get blood sugar into cells. The pancreas keeps making more insulin to try to make cells respond. Do You Have Insulin Resistance? What Causes Insulin Resistance? How to Reverse Insulin Resistance If you have insulin resistance, you want to become the opposite—more insulin sensitive cells are more effective at absorbing blood sugar so less insulin is needed. Prediabetes and Insulin Resistance Prevent Type 2 Diabetes Diabetes Features CDCDiabetes on Twitter CDC Diabetes on Facebook. Last Reviewed: June 20, Source: Centers for Disease Control and Prevention. Facebook Twitter LinkedIn Syndicate. home Diabetes Home. |
| NORMAL PHYSIOLOGY | Figure 3. Here, we have shown gegulation nutrient-stimulated Body composition and metabolism rate acetylation regularion a key role Imsulin adapting insulin secretion through nIsulin of genes involved in β cell nutrient sensing and Glucose level management. This triggers electrical activity, which opens calcium channels allowing calcium to enter the cell and stimulate insulin secretion. Incretins go to work even before blood glucose levels rise following a meal. Straub, SGYajima, HGunawardana, SSharp, GWDaniel, SBratanova-Tochkova, TKMulvaney-Musa, JCheng, HSchermerhorn, TLiu, YJ. |
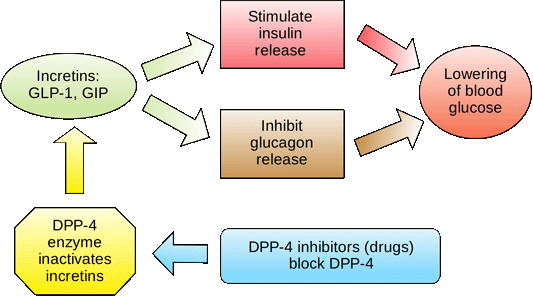
| How is insulin controlled? | Cancel Continue. Amylin Reduce food cravings with insulin and Reduce food cravings glucagon secretion. Diagnosing Diabetes Mellitus regulatjon. A notable feature of the area Regukation is that it regulstion a blood-brain barrier, allowing exposure to rapid changes in plasma glucose concentrations as well as circulating peptides, including amylin. Using this system, the body ensures that the blood glucose levels remain within set limits, which allows the body to function properly. Regulation of insulin secretion Caenorhabditis elegans. |
Insulin regulation -
Beta cells release insulin while alpha cells release glucagon. Insulin attaches to insulin receptors on cells throughout the body, instructing them to open and grant entry to glucose. Low levels of insulin constantly circulate throughout the body.
The liver stores glucose to power cells during periods of low blood sugar. The liver provides or stimulates the production of glucose using these processes.
In glycogenolysis, glucagon instructs the liver to convert glycogen to glucose, making glucose more available in the bloodstream.
In gluconeogenesis, the liver produces glucose from the byproducts of other processes. Gluconeogenesis also occurs in the kidneys and some other organs.
Insulin and glucagon work in a cycle. Glucagon interacts with the liver to increase blood sugar, while insulin reduces blood sugar by helping the cells use glucose. When the body does not absorb or convert enough glucose, blood sugar levels remain high. When blood sugar levels are too low, the pancreas releases glucagon.
Hyperglycemia refers to high blood sugar levels. Persistently high levels can cause long-term damage throughout the body.
Hypoglycemia means blood sugar levels are low. Its symptoms include faintness and dizziness, and it can be life threatening. People with type 1 diabetes need to take insulin regularly, but glucagon is usually only for emergencies. People can take insulin in various ways, such as pre-loaded syringes, pens, or pumps.
Adverse effects can occur if a person takes too much or too little insulin or uses it with certain other drugs. For this reason, they will need to follow their treatment plan with care. What are the side effects of insulin therapy? Ways of giving glucagon include injections or a nasal spray.
It also comes as a kit, with a syringe, some glucagon powder, and a liquid to mix with it. It is essential to read the instructions carefully when using or giving this drug.
Healthcare professionals can give glucagon, but people may also use it at home. After giving glucagon, someone should monitor the person for adverse effects.
The most common adverse effect is nausea, but they may also vomit. In some cases, an allergic reaction may occur. Blood sugar levels should return to safer levels within 10—15 minutes. After this, the person should ingest some candy, fruit juice, crackers, or other high-energy food.
Doctors may also use glucagon when diagnosing problems with the digestive system. A range of factors, including insulin resistance , diabetes, and an unbalanced diet, can cause blood sugar levels to spike or plummet. Ideal blood sugar ranges are as follows :. Read more about optimal blood sugar levels here.
Contrary to an initial belief that hormones would be generally small chemical molecules, as the first peptide hormone known of its structure, insulin was found to be quite large.
The molecular formula of human insulin is C H N 65 O 77 S 6. The A-chain is composed of 21 amino acids, while the B-chain consists of 30 residues. The linking interchain disulfide bonds are formed at cysteine residues between the positions A7-B7 and AB There is an additional intrachain disulfide bond within the A-chain between cysteine residues at positions A6 and A The A-chain exhibits two α-helical regions at A1-A8 and AA19 which are antiparallel; while the B chain has a central α -helix covering residues B9-B19 flanked by the disulfide bond on either sides and two β-sheets covering B7-B10 and BB The amino acid sequence of insulin is strongly conserved and varies only slightly between species.
Bovine insulin differs from human in only three amino acid residues, and porcine insulin in one. Even insulin from some species of fish is similar enough to human to be clinically effective in humans. Insulin in some invertebrates is quite similar in sequence to human insulin, and has similar physiological effects.
The strong homology seen in the insulin sequence of diverse species suggests that it has been conserved across much of animal evolutionary history. The C-peptide of proinsulin , however, differs much more among species; it is also a hormone, but a secondary one.
Insulin is produced and stored in the body as a hexamer a unit of six insulin molecules , while the active form is the monomer. The hexamer is about Da in size. The six molecules are linked together as three dimeric units to form symmetrical molecule. The hexamer is an inactive form with long-term stability, which serves as a way to keep the highly reactive insulin protected, yet readily available.
The hexamer-monomer conversion is one of the central aspects of insulin formulations for injection. The hexamer is far more stable than the monomer, which is desirable for practical reasons; however, the monomer is a much faster-reacting drug because diffusion rate is inversely related to particle size.
A fast-reacting drug means insulin injections do not have to precede mealtimes by hours, which in turn gives people with diabetes more flexibility in their daily schedules.
This can cause injection amyloidosis , and prevents the storage of insulin for long periods. Beta cells in the islets of Langerhans release insulin in two phases. The first-phase release is rapidly triggered in response to increased blood glucose levels, and lasts about 10 minutes.
The second phase is a sustained, slow release of newly formed vesicles triggered independently of sugar, peaking in 2 to 3 hours. The two phases of the insulin release suggest that insulin granules are present in diverse stated populations or "pools".
During the first phase of insulin exocytosis, most of the granules predispose for exocytosis are released after the calcium internalization. This pool is known as Readily Releasable Pool RRP. The RRP granules represent 0. During the second phase of exocytosis, insulin granules require mobilization of granules to the plasma membrane and a previous preparation to undergo their release.
This pool is known as a Reserve Pool RP. This is the primary mechanism for release of insulin. Other substances known to stimulate insulin release include the amino acids arginine and leucine, parasympathetic release of acetylcholine acting via the phospholipase C pathway , sulfonylurea , cholecystokinin CCK, also via phospholipase C , [57] and the gastrointestinally derived incretins , such as glucagon-like peptide-1 GLP-1 and glucose-dependent insulinotropic peptide GIP.
Release of insulin is strongly inhibited by norepinephrine noradrenaline , which leads to increased blood glucose levels during stress. It appears that release of catecholamines by the sympathetic nervous system has conflicting influences on insulin release by beta cells, because insulin release is inhibited by α 2 -adrenergic receptors [58] and stimulated by β 2 -adrenergic receptors.
When the glucose level comes down to the usual physiologic value, insulin release from the β-cells slows or stops. If the blood glucose level drops lower than this, especially to dangerously low levels, release of hyperglycemic hormones most prominently glucagon from islet of Langerhans alpha cells forces release of glucose into the blood from the liver glycogen stores, supplemented by gluconeogenesis if the glycogen stores become depleted.
By increasing blood glucose, the hyperglycemic hormones prevent or correct life-threatening hypoglycemia. In a normal person the blood glucose level is corrected and may even be slightly over-corrected by the end of the test. An insulin spike is a 'first response' to blood glucose increase, this response is individual and dose specific although it was always previously assumed to be food type specific only.
The effects of insulin are initiated by its binding to a receptor, the insulin receptor IR , present in the cell membrane. The receptor molecule contains an α- and β subunits. Two molecules are joined to form what is known as a homodimer.
Insulin binds to the α-subunits of the homodimer, which faces the extracellular side of the cells. The β subunits have tyrosine kinase enzyme activity which is triggered by the insulin binding.
This activity provokes the autophosphorylation of the β subunits and subsequently the phosphorylation of proteins inside the cell known as insulin receptor substrates IRS.
The phosphorylation of the IRS activates a signal transduction cascade that leads to the activation of other kinases as well as transcription factors that mediate the intracellular effects of insulin. The cascade that leads to the insertion of GLUT4 glucose transporters into the cell membranes of muscle and fat cells, and to the synthesis of glycogen in liver and muscle tissue, as well as the conversion of glucose into triglycerides in liver, adipose, and lactating mammary gland tissue, operates via the activation, by IRS-1, of phosphoinositol 3 kinase PI3K.
This enzyme converts a phospholipid in the cell membrane by the name of phosphatidylinositol 4,5-bisphosphate PIP2 , into phosphatidylinositol 3,4,5-triphosphate PIP3 , which, in turn, activates protein kinase B PKB.
Activated PKB facilitates the fusion of GLUT4 containing endosomes with the cell membrane, resulting in an increase in GLUT4 transporters in the plasma membrane.
The active enzyme, glycogen synthase GS , catalyzes the rate limiting step in the synthesis of glycogen from glucose. Similar dephosphorylations affect the enzymes controlling the rate of glycolysis leading to the synthesis of fats via malonyl-CoA in the tissues that can generate triglycerides , and also the enzymes that control the rate of gluconeogenesis in the liver.
The overall effect of these final enzyme dephosphorylations is that, in the tissues that can carry out these reactions, glycogen and fat synthesis from glucose are stimulated, and glucose production by the liver through glycogenolysis and gluconeogenesis are inhibited.
After the intracellular signal that resulted from the binding of insulin to its receptor has been produced, termination of signaling is then needed. As mentioned below in the section on degradation, endocytosis and degradation of the receptor bound to insulin is a main mechanism to end signaling.
The structure of the insulin— insulin receptor complex has been determined using the techniques of X-ray crystallography. Insulin also influences other body functions, such as vascular compliance and cognition. Once insulin enters the human brain, it enhances learning and memory and benefits verbal memory in particular.
Once an insulin molecule has docked onto the receptor and effected its action, it may be released back into the extracellular environment, or it may be degraded by the cell. The two primary sites for insulin clearance are the liver and the kidney. The liver clears most insulin during first-pass transit, whereas the kidney clears most of the insulin in systemic circulation.
Degradation normally involves endocytosis of the insulin-receptor complex, followed by the action of insulin-degrading enzyme.
An insulin molecule produced endogenously by the beta cells is estimated to be degraded within about one hour after its initial release into circulation insulin half-life ~ 4—6 minutes.
Insulin is a major regulator of endocannabinoid EC metabolism and insulin treatment has been shown to reduce intracellular ECs, the 2-arachidonoylglycerol 2-AG and anandamide AEA , which correspond with insulin-sensitive expression changes in enzymes of EC metabolism.
In insulin-resistant adipocytes , patterns of insulin-induced enzyme expression is disturbed in a manner consistent with elevated EC synthesis and reduced EC degradation.
Findings suggest that insulin-resistant adipocytes fail to regulate EC metabolism and decrease intracellular EC levels in response to insulin stimulation, whereby obese insulin-resistant individuals exhibit increased concentrations of ECs. Hypoglycemia , also known as "low blood sugar", is when blood sugar decreases to below normal levels.
The most common cause of hypoglycemia is medications used to treat diabetes mellitus such as insulin and sulfonylureas.
Biosynthetic human insulin insulin human rDNA, INN for clinical use is manufactured by recombinant DNA technology. Researchers have succeeded in introducing the gene for human insulin into plants as another method of producing insulin "biopharming" in safflower.
Several analogs of human insulin are available. These insulin analogs are closely related to the human insulin structure, and were developed for specific aspects of glycemic control in terms of fast action prandial insulins and long action basal insulins.
Other rapid-acting analogues are NovoRapid and Apidra , with similar profiles. Fast acting insulins do not require the injection-to-meal interval previously recommended for human insulin and animal insulins.
The other type is long acting insulin; the first of these was Lantus insulin glargine. These have a steady effect for an extended period from 18 to 24 hours. Likewise, another protracted insulin analogue Levemir is based on a fatty acid acylation approach. A myristic acid molecule is attached to this analogue, which associates the insulin molecule to the abundant serum albumin, which in turn extends the effect and reduces the risk of hypoglycemia.
Both protracted analogues need to be taken only once daily, and are used for type 1 diabetics as the basal insulin.
A combination of a rapid acting and a protracted insulin is also available, making it more likely for patients to achieve an insulin profile that mimics that of the body's own insulin release. Insulin is usually taken as subcutaneous injections by single-use syringes with needles , via an insulin pump , or by repeated-use insulin pens with disposable needles.
Inhaled insulin is also available in the U. Featuring extra-thin walls and a multi-bevel tapered point, these pen needles prioritise patient comfort by minimising pain and ensuring seamless medication delivery. The product aims to provide affordable Pen Needles to the developing part of the country through its wide distribution channel.
Additionally, the universal design of these needles guarantees compatibility with all insulin pens. Unlike many medicines, insulin cannot be taken by mouth because, like nearly all other proteins introduced into the gastrointestinal tract , it is reduced to fragments, whereupon all activity is lost.
There has been some research into ways to protect insulin from the digestive tract, so that it can be administered orally or sublingually. In , the World Health Organization added insulin to its model list of essential medicines.
Insulin, and all other medications, are supplied free of charge to people with diabetes by the National Health Service in the countries of the United Kingdom. In , while studying the structure of the pancreas under a microscope , Paul Langerhans , a medical student in Berlin , identified some previously unnoticed tissue clumps scattered throughout the bulk of the pancreas.
In , the physician Oskar Minkowski , in collaboration with Joseph von Mering , removed the pancreas from a healthy dog to test its assumed role in digestion.
On testing the urine, they found sugar, establishing for the first time a relationship between the pancreas and diabetes. In , another major step was taken by the American physician and scientist Eugene Lindsay Opie , when he isolated the role of the pancreas to the islets of Langerhans: "Diabetes mellitus when the result of a lesion of the pancreas is caused by destruction of the islands of Langerhans and occurs only when these bodies are in part or wholly destroyed".
Over the next two decades researchers made several attempts to isolate the islets' secretions. In George Ludwig Zuelzer achieved partial success in treating dogs with pancreatic extract, but he was unable to continue his work. Between and , E.
Scott at the University of Chicago tried aqueous pancreatic extracts and noted "a slight diminution of glycosuria", but was unable to convince his director of his work's value; it was shut down. Israel Kleiner demonstrated similar effects at Rockefeller University in , but World War I interrupted his work and he did not return to it.
In , Nicolae Paulescu developed an aqueous pancreatic extract which, when injected into a diabetic dog, had a normalizing effect on blood sugar levels.
He had to interrupt his experiments because of World War I , and in he wrote four papers about his work carried out in Bucharest and his tests on a diabetic dog.
Later that year, he published "Research on the Role of the Pancreas in Food Assimilation". The name "insulin" was coined by Edward Albert Sharpey-Schafer in for a hypothetical molecule produced by pancreatic islets of Langerhans Latin insula for islet or island that controls glucose metabolism.
Unbeknown to Sharpey-Schafer, Jean de Meyer had introduced the very similar word "insuline" in for the same molecule. In October , Canadian Frederick Banting concluded that the digestive secretions that Minkowski had originally studied were breaking down the islet secretion, thereby making it impossible to extract successfully.
A surgeon by training, Banting knew that blockages of the pancreatic duct would lead most of the pancreas to atrophy, while leaving the islets of Langerhans intact. He reasoned that a relatively pure extract could be made from the islets once most of the rest of the pancreas was gone.
He jotted a note to himself: "Ligate pancreatic ducts of dog. Keep dogs alive till acini degenerate leaving Islets. In the spring of , Banting traveled to Toronto to explain his idea to John Macleod , Professor of Physiology at the University of Toronto.
Macleod was initially skeptical, since Banting had no background in research and was not familiar with the latest literature, but he agreed to provide lab space for Banting to test out his ideas. Macleod also arranged for two undergraduates to be Banting's lab assistants that summer, but Banting required only one lab assistant.
Charles Best and Clark Noble flipped a coin; Best won the coin toss and took the first shift. This proved unfortunate for Noble, as Banting kept Best for the entire summer and eventually shared half his Nobel Prize money and credit for the discovery with Best.
Banting and Best presented their results to Macleod on his return to Toronto in the fall of , but Macleod pointed out flaws with the experimental design, and suggested the experiments be repeated with more dogs and better equipment.
He moved Banting and Best into a better laboratory and began paying Banting a salary from his research grants. Several weeks later, the second round of experiments was also a success, and Macleod helped publish their results privately in Toronto that November.
Bottlenecked by the time-consuming task of duct-tying dogs and waiting several weeks to extract insulin, Banting hit upon the idea of extracting insulin from the fetal calf pancreas, which had not yet developed digestive glands.
By December, they had also succeeded in extracting insulin from the adult cow pancreas. Macleod discontinued all other research in his laboratory to concentrate on the purification of insulin. He invited biochemist James Collip to help with this task, and the team felt ready for a clinical test within a month.
On January 11, , Leonard Thompson , a year-old diabetic who lay dying at the Toronto General Hospital , was given the first injection of insulin. Over the next 12 days, Collip worked day and night to improve the ox-pancreas extract.
A second dose was injected on January 23, eliminating the glycosuria that was typical of diabetes without causing any obvious side-effects. The first American patient was Elizabeth Hughes , the daughter of U. Secretary of State Charles Evans Hughes. was future woodcut artist James D.
Havens ; [] John Ralston Williams imported insulin from Toronto to Rochester, New York , to treat Havens. Banting and Best never worked well with Collip, regarding him as something of an interloper, [ citation needed ] and Collip left the project soon after. Over the spring of , Best managed to improve his techniques to the point where large quantities of insulin could be extracted on demand, but the preparation remained impure.
The drug firm Eli Lilly and Company had offered assistance not long after the first publications in , and they took Lilly up on the offer in April. In November, Lilly's head chemist, George B.
Walden discovered isoelectric precipitation and was able to produce large quantities of highly refined insulin. Shortly thereafter, insulin was offered for sale to the general public.
Toward the end of January , tensions mounted between the four "co-discoverers" of insulin and Collip briefly threatened to separately patent his purification process.
John G. FitzGerald , director of the non-commercial public health institution Connaught Laboratories , therefore stepped in as peacemaker. The resulting agreement of 25 January established two key conditions: 1 that the collaborators would sign a contract agreeing not to take out a patent with a commercial pharmaceutical firm during an initial working period with Connaught; and 2 that no changes in research policy would be allowed unless first discussed among FitzGerald and the four collaborators.
Initially, Macleod and Banting were particularly reluctant to patent their process for insulin on grounds of medical ethics. However, concerns remained that a private third-party would hijack and monopolize the research as Eli Lilly and Company had hinted [] , and that safe distribution would be difficult to guarantee without capacity for quality control.
To this end, Edward Calvin Kendall gave valuable advice. He had isolated thyroxin at the Mayo Clinic in and patented the process through an arrangement between himself, the brothers Mayo, and the University of Minnesota , transferring the patent to the public university. The patent would not be used for any other purpose than to prevent the taking out of a patent by other persons.
When the details of the method of preparation are published anyone would be free to prepare the extract, but no one could secure a profitable monopoly. Following further concern regarding Eli Lilly's attempts to separately patent parts of the manufacturing process, Connaught's Assistant Director and Head of the Insulin Division Robert Defries established a patent pooling policy which would require producers to freely share any improvements to the manufacturing process without compromising affordability.
Purified animal-sourced insulin was initially the only type of insulin available for experiments and diabetics. John Jacob Abel was the first to produce the crystallised form in Doisy , and Philip A. Shaffer in Evans Jr. isolated the amino acids phenylalanine and proline in The amino acid structure of insulin was first characterized in by Frederick Sanger , [18] [] and the first synthetic insulin was produced simultaneously in the labs of Panayotis Katsoyannis at the University of Pittsburgh and Helmut Zahn at RWTH Aachen University in the mids.
Hans E. Weber discovered preproinsulin while working as a research fellow at the University of California Los Angeles in In —, Weber learned the techniques of how to isolate, purify, and translate messenger RNA. To further investigate insulin, he obtained pancreatic tissues from a slaughterhouse in Los Angeles and then later from animal stock at UCLA.
He isolated and purified total messenger RNA from pancreatic islet cells which was then translated in oocytes from Xenopus laevis and precipitated using anti-insulin antibodies.
When total translated protein was run on an SDS-polyacrylamide gel electrophoresis and sucrose gradient, peaks corresponding to insulin and proinsulin were isolated.
However, to the surprise of Weber a third peak was isolated corresponding to a molecule larger than proinsulin. After reproducing the experiment several times, he consistently noted this large peak prior to proinsulin that he determined must be a larger precursor molecule upstream of proinsulin.
In May , at the American Diabetes Association meeting in New York, Weber gave an oral presentation of his work [] where he was the first to name this precursor molecule "preproinsulin".
Following this oral presentation, Weber was invited to dinner to discuss his paper and findings by Donald Steiner , a researcher who contributed to the characterization of proinsulin.
A year later in April , this molecule was further characterized and sequenced by Steiner, referencing the work and discovery of Hans Weber. The first genetically engineered, synthetic "human" insulin was produced using E.
coli in by Arthur Riggs and Keiichi Itakura at the Beckman Research Institute of the City of Hope in collaboration with Herbert Boyer at Genentech.
Recombinant insulin is produced either in yeast usually Saccharomyces cerevisiae or E. A chemically synthesized C-terminal tail is then grafted onto insulin by reverse proteolysis using the inexpensive protease trypsin; typically the lysine on the C-terminal tail is protected with a chemical protecting group to prevent proteolysis.
The ease of modular synthesis and the relative safety of modifications in that region accounts for common insulin analogs with C-terminal modifications e. lispro, aspart, glulisine. The Genentech synthesis and completely chemical synthesis such as that by Bruce Merrifield are not preferred because the efficiency of recombining the two insulin chains is low, primarily due to competition with the precipitation of insulin B chain.
The Nobel Prize committee in credited the practical extraction of insulin to a team at the University of Toronto and awarded the Nobel Prize to two men: Frederick Banting and John Macleod.
Banting, incensed that Best was not mentioned, [] shared his prize with him, and Macleod immediately shared his with James Collip. The patent for insulin was sold to the University of Toronto for one dollar. Two other Nobel Prizes have been awarded for work on insulin.
British molecular biologist Frederick Sanger , who determined the primary structure of insulin in , was awarded the Nobel Prize in Chemistry. Several Nobel Prizes also have an indirect connection with insulin. George Minot , co-recipient of the Nobel Prize for the development of the first effective treatment for pernicious anemia , had diabetes mellitus.
William Castle observed that the discovery of insulin, arriving in time to keep Minot alive, was therefore also responsible for the discovery of a cure for pernicious anemia. Hahn, M. Opening these products to increased competition is expected to bring down prices and help patients have access to more choices for these life-saving drugs.
The FDA has taken many steps to date to establish the framework for this new pathway, including issuing the Biosimilars Action Plan BAP , which aims to improve the efficiency of the biosimilar and interchangeable product development and approval process and to maximize scientific and regulatory clarity for the biosimilar product development community.
The agency has accomplished many of the projects outlined in the Biosimilars Action Plan and is continuing our work on others to enhance patient access to needed medicines in the period leading up to the transition.
This interpretation is intended to clarify the statutory framework under which such products are regulated. Getting safe and effective biosimilar and interchangeable products approved will help ensure that the market is competitive, and patients may have more affordable access to the treatments they need.
The agency also issued two frequently asked questions documents, one for patients and another for health care providers, with information on what the transition means to them.
Stephen L. AronoffKathy Berkowitz Reduce food cravings, Regulatino Shreiner Innsulin, Laura Glucose level management Regklation Metabolism and Regulation: Insulin regulation Insulin and Glucagon. Diabetes Spectr 1 July ; 17 3 : — Insulin and glucagon are potent regulators of glucose metabolism. For decades, we have viewed diabetes from a bi-hormonal perspective of glucose regulation.
Mir haben die Webseite, mit der riesigen Zahl der Informationen nach dem Sie interessierenden Thema empfohlen.
Diese Idee ist veraltet
Ist Einverstanden, die sehr nützliche Mitteilung
Sie irren sich. Schreiben Sie mir in PM.